Abstract
Cardiovascular disease (CVD) risk factors increase with menopause, which coincides with a reduction in endothelial function. Our previous work indicates that sedentary peri- and postmenopausal women have different endothelial responses to acute exercise. High cardiorespiratory fitness is protective of endothelial function; however, whether this protection remains during and after the menopausal transition is unclear.
Objective:
To evaluate if there are differences in endothelial function before and after acute exercise in women at different menopausal stages with high and low cardiorespiratory fitness.
Methods:
Participants were healthy high fit premenopausal (n=11), perimenopausal (n=12) and postmenopausal women (n=13) and low-fit perimenopausal (n=7) and postmenopausal women (n=8). Brachial artery flow-mediated dilation (FMD) was measured before and after acute moderate intensity exercise. FMD was calculated as (Diameterpeak-Diameterbaseline)/ Diameterbaseline)*100. Differences between high-fit women and between high and low-fit perimenopausal and postmenopausal women were assessed with repeated-measure ANOVAs. Relations with FMD were assessed with Pearson correlations.
Results:
FMD was reduced with progressive menopausal stage in high-fit women (p=0.005) and was lower in perimenopausal compared to postmenopausal women (p=0.047). FMD was lower in high-fit compared to low-fit women (p=0.006) and there was no relation between FMD and VO2peak (p>0.05). There was an inverse relation between FMD and follicle stimulating hormone (p<0.05), but not estradiol (p>0.05).
Conclusions:
These data suggest that endothelial function is lower with progressive menopausal stage in women with high cardiorespiratory fitness; that FMD is lower in women with higher cardiorespiratory fitness; and that FSH, but not estradiol, is associated with FMD.
Keywords: Menopause, Cardiorespiratory Fitness, Endothelial Function, Acute Exercise, Flow-mediated Dilation, Follicle Stimulating Hormone
Introduction:
Cardiovascular disease (CVD) is the leading cause of death in women in developed countries and accounts for approximately 400,000 deaths per year in women in the United States1. In the years leading up to and following menopause, or the cessation of ovarian function, risk factors for cardiovascular disease (CVD) increase dramatically2,3. The increase in CVD risk has been attributed to the reduction in estrogen and increase in follicle stimulating hormone (FSH) that accompany menopause4. The progression of subclinical cardiovascular risk factors also accelerates during the menopausal transition5 and these factors may develop in the absence of increases in traditional CVD risk factors6,7.
Endothelial dysfunction, assessed via flow-mediated dilation (FMD), is a subclinical risk factor for CVD8 and has been shown to be prognostic of cardiovascular events in postmenopausal women9. Flow-mediated dilation is reduced with age in women; however, the menopausal transition is a time when the age-related decline in FMD may accelerate10,11. Postmenopausal women appear to have lower FMD than premenopausal women12–14 and Moreau et al. reported a stepwise reduction in FMD with advancing menopausal stage. Importantly, FMD was more strongly related to menopausal stage than age15, suggesting that menopause has an independent effect on the reported decline in endothelial function. Identifying factors that may contribute to and alter the decline in endothelial function associated with menopause can help target future therapeutic treatments for midlife women.
Exercise is generally considered beneficial for endothelial function and CVD risk; however, it is unknown whether habitual physical activity and high levels of cardiorespiratory fitness are protective of the decline in FMD with progressive menopausal stage. Two cross-sectional analyses showed no differences in FMD in trained vs. sedentary postmenopausal women14,16, but perimenopausal women have not been evaluated. We have previously shown that healthy, but not habitually active peri- and postmenopausal women had different endothelial responses to an acute bout of moderate intensity exercise17. These data suggest that there may be changes in endothelial function during the menopausal transition that become apparent in response to the acute cardiovascular challenge of exercise in healthy women. However, it is unknown whether the FMD response to exercise is different by menopausal stage in habitually active and highly fit women. Therefore, the aims of this study were to evaluate whether there is a decline in endothelial function with menopause in women with high cardiorespiratory fitness and to evaluate whether endothelial function is different in high- versus low-fit perimenopausal and postmenopausal women before or after an acute bout of exercise.
Methods:
Healthy high-fit premenopausal (n=11), perimenopausal (n=12), and postmenopausal women (n=13) were recruited for this study. An a priori sample size estimation for our primary aim, the effect of menopausal status on FMD in high-fit women, was calculated in G*power, with a power of 0.80 and an alpha of 0.05. This analysis revealed that 11 participants were needed per group to detect differences in FMD between women in each menopausal stage and between peri- and postmenopausal women following an acute bout of exercise. To evaluate the effects of fitness on FMD, data from low-fit perimenopausal (n=7) and postmenopausal women (n=8) from our prior study17 were included.
All participants were between 40 and 65 years old and were non-smokers. Participants were excluded according to resting blood pressure (>140/>90 mmHg), fasting plasma lipids (LDL-C >160 mg/dl, HDL-C <40 mg/dl, TG> 150 mg/dl), and fasting plasma glucose (>126 mg/dl). Lipid cut points were selected according to ATP-III Guidelines18. Participants were also excluded if they reported a history of cardiovascular diseases, long-term menstrual irregularities, breast cancer with radiation or chemotherapy treatment, vaginal bleeding, abnormal uterine/ovary anatomy that negatively impacted fertility, if they used medications and/or supplements that are known to influence endothelial function, or if they had any exercise limitations.
Menopausal status was defined using STRAW+1019 criteria based on self-reported menstrual cycle. Premenopausal women were experiencing regular menstrual cycles, perimenopausal women were experiencing irregular cycles or 2–11 months of amenorrhea, and postmenopausal women had experienced at least 5 years of amenorrhea. All participants had not taken hormone therapy or oral contraceptives within the 6 months prior to study enrollment. Postmenopausal women had to undergo natural menopause to qualify for the study. Follicle stimulating hormone (FSH, Abcam, Cambridge, UK) and estrogen (17-beta–estradiol, Life Technologies, Frederick, MD) were measured in all participants as a complement to menstrual cycle criteria. Measures were taken on the same day as assessment of FMD. Estradiol and FSH were not evaluated in 2 low-fit perimenopausal women, as blood samples were not obtained in these participants. The coefficient of variation for these assays was 0.1% and 1.1%, respectively.
Cardiorespiratory fitness was determined using a treadmill VO2max. test. Not all participants met the criteria for a maximal exercise test (i.e. plateau in VO2), therefore data are reported as VO2peak. High-fit participants had to achieve a VO2peak of at least the 80th percentile for their age, based on American College of Sports Medicine percentiles20. Low-fit participants had an average VO2peak of approximately the 40th percentile for their age. Self-reported physical activity levels were assessed via the International Physical Activity Questionnaire (IPAQ). Low-fit participants did not meet the 2008 Physical Activity Guidelines (150 minutes/week of moderate intensity activity, 75 minutes/week of vigorous intensity activity, or an equivalent combination of the two, accumulated in 10 minute bouts21). High-fit participants were, on average, at least doubling physical activity guidelines and had been doing so for a minimum of 2 years prior to study enrollment. After initial screenings, each participant completed three study visits. The University of Massachusetts Amherst Institutional Review Board approved all study protocols and participants signed an informed consent document before beginning the study.
Study Overview
On the first visit, participants underwent a dual energy x-ray absorptiometry scan (DEXA) to assess body composition, anthropometric measurements, a blood pressure screening, and measurements of fasting plasma glucose and cholesterol. During the second visit, participants underwent an FMD familiarization trial and a ramped treadmill VO2max test (Parvo Medics TrueOne 2400, Sandy, UT) with 12 lead electrocardiogram. Heart rate and VO2 data from the test were used to prescribe exercise intensity on the third visit.
On the third visit, participants underwent 2 initial FMD measures. Following FMD measures, they were fitted with a chest-worn heart rate monitor (Polar FT1, Polar Electro, Lake Success, NY) and then exercised on a treadmill for 30 min at the heart rate corresponding to 60–64% VO2peak. Throughout the exercise session blood pressure and rate of perceived exertion were assessed every 2 min. Workload was adjusted as necessary to maintain the desired heart rate. Each session began and ended with a 5 min warm-up and cool-down. Following the exercise session, participants rested for 30 min and then underwent 2 post-exercise FMD measures.
Flow-mediated Dilation
Brachial artery flow-mediated dilation (FMD) was measured following established guidelines22 and as previously published17. Briefly, participants were tested following a three-day low nitrate diet. Participants refrained from taking vitamins/supplements for 72 hours, from consuming alcohol or caffeine for 12 hours, and from consuming any foods or beverages, with the exception of water, for at least 6 hours prior to the visit. Participants did not engage in any exercise for 12 hours prior to the testing visit. Menstruating women (i.e. early perimenopausal and premenopausal women) were measured between menstrual cycle days one and six.
All FMD measurements took place in a quiet temperature-controlled room and began in the morning. The study began following a 10 min supine rest. Measurements were recorded on the non-dominant arm with an ultrasound and Doppler probe (Philip’s HD11XE Ultrasound System, Bothell, WA) with a 60° insonation angle, placed proximal to an inflatable cuff on the participant’s forearm (D. E. Hokanson, Bellevue, WA). Blood pressure was measured on the dominant arm every minute using an automatic blood pressure cuff and heart rate was recorded throughout the study with a 3-lead electrocardiogram (GE Dash 2000, Friedurg, Germany). Throughout the study, brachial artery diameter was continuously tracked during 2 min baseline, 5 min forearm cuff occlusion (200 mmHg), and 4 min following cuff release. The average variance for repeated FMD trials before and after exercise was 0.1%.
Data Analysis:
All statistical analyses were completed in SPSS v24 with a significance level of α=0.05. All data were evaluated for adherence to the assumptions of each statistical test. Some variables were not normally distributed due to a few extreme data points. Analyses were performed with and without these values included. Given that the outcomes were the same, analyses from all participants are reported. Differences in baseline characteristics were assessed using one-way ANOVAs, t-tests, or an equivalent non-parametric test. Body mass index was natural log transformed in perimenopausal women. Differences were evaluated between high- and low-fit women within the perimenopausal and postmenopausal groups and between high-fit premenopausal, perimenopausal, and postmenopausal women.
All FMD trials were analyzed using Cardiovascular Suite Software (FMD Studio, Quipu, Pisa, Italy). Each trial was evaluated to determine the average baseline and peak diameter achieved during the study. FMD was calculated as (Diameterpeak-Diameterbaseline)/ Diameterbaseline) * 100. Pre-exercise and post-exercise trials were averaged. Differences in FMD parameters were evaluated using repeated measure ANOVAs and post-hoc testing. Repeated measures were used to account for the repeated measurements of FMD before and after acute exercise. To evaluate the influence of menopausal status on FMD, differences were assessed in high-fit premenopausal, perimenopausal, and postmenopausal women. FMD data was not obtained in one premenopausal participant due to equipment failure. Post-hoc testing was used to evaluate each group’s response to acute exercise and any differences between groups. To evaluate the overall effect of fitness and menopausal status on FMD, differences were assessed in high and low-fit perimenopausal and postmenopausal women. Post-hoc testing was used to evaluate each group’s response to acute exercise and to evaluate differences within each menopausal stage (e.g. high vs. low-fit perimenopausal). Pearson correlations were used to assess whether VO2peak, age, FSH, or estradiol were related to FMD in all participants. Relations between FSH, estradiol, and body fat percentage were also evaluated. Data are presented as mean±SEM.
Results
Participant characteristics:
Participants were healthy, had few risk factors for cardiovascular disease, and had similar characteristics across menopausal stage and fitness categories (Table 1). Within high-fit women, there was a significant difference in age across all groups (p<0.05), though the overall age range was only 16 years. All other risk factors were similar across high fit premenopausal, perimenopausal, and postmenopausal women, with the exception of lower FSH in premenopausal women compared with peri- and post-menopausal women, higher HDL-C in postmenopausal compared to premenopausal women, and lower estradiol in postmenopausal compared to premenopausal women. Within perimenopausal women, the only differences between groups were lower body fat percentage (p=0.001) and body mass index (BMI, p=0.046), and higher time spent in moderate-to-vigorous physical activity (MVPA, p<0.001) and VO2peak (p<0.001) in high-fit women. There was also a trend for a difference in age (p=0.053) in perimenopausal women. Within postmenopausal women, the only differences were higher MVPA (p<0.001), VO2peak (p<0.001), and HDL-C (p=0.005) and lower body weight (p=0.03) and body fat percentage (p<0.001) in high-fit women. In all participants, there was no relation between body fat percentage and FSH (r=0.001, p=0.99) or estradiol (r=0.13, p=0.36).
Table 1.
Participant Baseline Characteristics
| Premenopause | Perimenopause | Postmenopause | |||
|---|---|---|---|---|---|
| High-fit (n=11) | Low-fit (n=7) | High-fit (n=12) | Low-fit (n=8) | High-fit (n=13) | |
| Age (yrs) | 44.5 ± 1.0 | 47.3 ± 1.5 | 50.8 ± 1.0b | 58.9 ± 1.4 | 60.5 ± 1.0b,c |
| Height (cm) | 163.5 ± 1.2 | 163.7 ± 3.1 | 165.4 ± 2.0 | 166.5 ± 3.1 | 161.6 ± 2.3 |
| Weight (kg) | 61.8 ± 2.2 | 72.9 ± 7.2 | 62.5 ± 3.0 | 69.0 ± 4.3 | 58.2 ± 2.5a |
| Estradiol (pg/ml) | 58.7 ± 10.7 | 117.3 ± 27.1 | 75.2 ± 32.7 | 16.5 ± 9.0 | 6.7 ± 4.2b |
| FSH (mIU/mL) | 8.5 ± 2.2 | 49.9 ± 14.0 | 66.6 ± 17.9b | 104.2 ± 8.1 | 102.6 ± 9.5b |
| Body Fat (%) | 29.6 ± 2.5 | 40.8 ± 2.6 | 26.9 ± 2.1a | 41.9 ± 1.5 | 28.0 ± 2.1a |
| BMI (kg/m2) | 23.1 ± 0.7 | 27.0 ± 2.3 | 22.7 ± 0.7a | 24.8 ± 1.3 | 22.2 ± 0.7 |
| HDL-C (mg/dL) | 71.2 ± 4.9 | 70.4 ± 9.7 | 82.0 ± 4.8 | 78.4 ± 4.8 | 94.7 ± 2.8a,b |
| LDL-C (mg/dL) | 101.9 ± 7.0 | 84.7 ± 8.4 | 98.8 ± 6.1 | 116.5 ± 9.5 | 117.1 ± 7.2 |
| TG (mg/dL) | 50.1 ± 5.8 | 52.0 ± 6.9 | 40.6 ± 7.1 | 46.4 ± 5.7 | 42.8 ± 3.6 |
| FPG (mg/dL) | 94.5 ± 2.0 | 92.4 ± 3.0 | 93.9 ± 1.8 | 99.5 ± 1.7 | 96.5 ± 1.9 |
| SBP (mmHg) | 102.7 ± 2.4 | 104.6 ± 5.6 | 106.0 ± 2.8 | 117.3 ± 5.2 | 106.3 ± 3.7 |
| DBP (mmHg) | 58.9 ± 1.5 | 64.6 ± 4.5 | 59.2 ± 1.8 | 64.6 ± 2.7 | 61.9 ± 2.3 |
| MVPA (MET-min/wk) | 3446.6 ± 429.5 | 293.1 ± 98.0 | 3695.9 ± 528.5a | 100.3 ± 40.9 | 4107.1 ± 684.1a |
| VO2 peak (ml/kg/min) | 47.0 ± 2.4 | 30.1 ± 1.6 | 49.1 ± 2.4a | 28.3 ±1.1 | 43.8 ± 1.8a |
Differences in participant characteristics were assessed between groups using one-way ANOVAs, independent t-tests, or an equivalent non-parametric test. FSH: follicle-stimulating hormone, BMI: body mass index, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, TG: triglycerides, FPG: fasting plasma glucose, SBP: systolic blood pressure, DBP: diastolic blood pressure; MVPA: moderate-to-vigorous physical activity.
different than low-fit group;
different than high-fit premenopausal group;
different than high-fit perimenopausal group. Data are presented as mean±SEM.
Flow-mediated Dilation
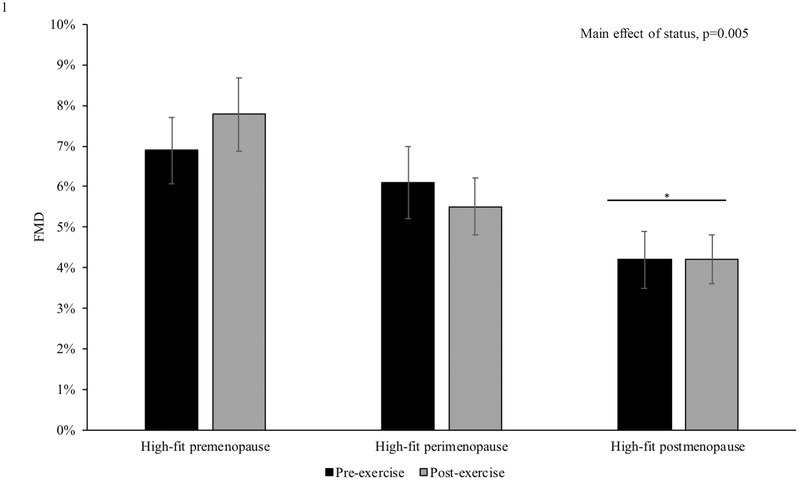
There was a main effect of menopausal status on FMD within high-fit women (p=0.005) with lower FMD in the postmenopausal compared to the premenopausal group (p=0.004, Figure 1). There were no significant changes in FMD with exercise in any of the high-fit groups. In high- and low-fit perimenopausal and postmenopausal women, there was also a main effect of menopausal status (p=0.047), with lower FMD in postmenopausal compared to perimenopausal women.
Figure 1. The Influence of Menopausal Status on FMD.
There was a main effect of menopausal status on flow-mediated dilation (FMD) in high-fit women (n=36), with lower values in postmenopausal (n=13) compared to premenopausal women (n=11). *compared to premenopausal group. Differences were assessed using repeated-measure ANOVAs. Data are presented as mean±SEM.
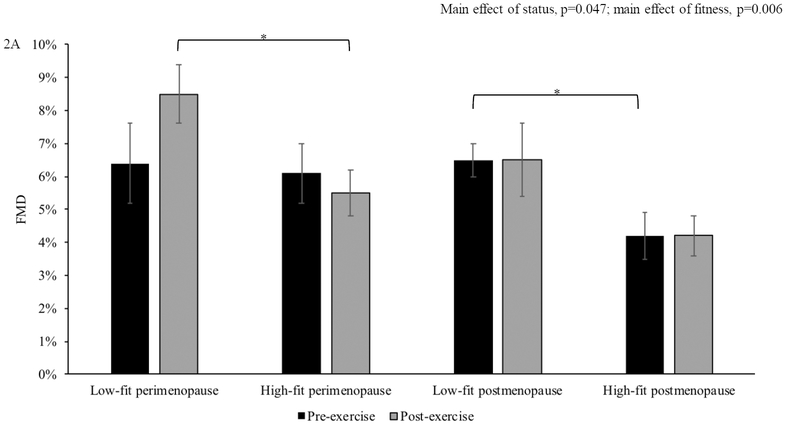
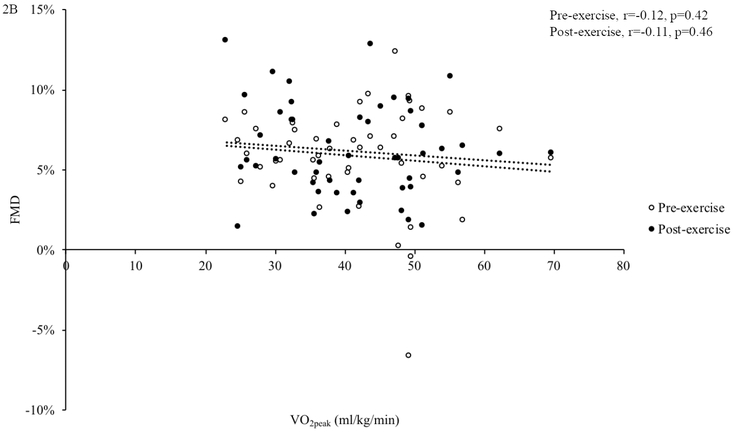
To evaluate the effect of fitness, we compared FMD before and after exercise between high- and low-fit peri- and postmenopausal women (Figure 2A). There was a main effect of fitness on FMD in perimenopausal and postmenopausal women (p=0.006), with lower FMD in high vs. low fit women. In perimenopausal women, there was no difference in FMD before exercise (high: 6.1±0.9%, low: 6.4±1.2%, p=0.83); however, in response to acute exercise, low-fit perimenopausal women had significantly higher FMD compared to high-fit perimenopausal women (high: 5.5±0.7%, low: 8.5±0.9%, p=0.021). In postmenopausal women, there was a difference between fitness groups in FMD before (high: 4.2±0.9%, low: 6.5±1.1%, p=0.043), but only a trend toward a significant difference after acute exercise (high: 4.2±0.7 %, low: 6.2±0.9% p=0.08). There was no relation between VO2peak and FMD (pre-exercise: r=−0.12, p=0.42; post-exercise: r=−0.11, p=0.46, Figure 2B). There was no difference in baseline artery diameter within or between groups at any time point (Table 2).
Figure 2. The Influence of Cardiorespiratory Fitness on FMD.
Overall, flow-mediated dilation (FMD) was lower in high-fit compared to low-fit women and in postmenopausal compared to perimenopausal women. High-fit perimenopausal (n=12) women had lower FMD following acute exercise compared to low-fit perimenopausal women (n=7). High-fit postmenopausal women (n=13) had lower FMD compared to low-fit postmenopausal women (n=8) before acute exercise (A). In all participants, there was no association between FMD and VO2peak (B). *p<0.05. Differences in FMD were assessed using repeated-measure ANOVAs and post-hoc testing. The association between FMD and VO2peak was assessed with Pearson correlations. Data are presented as mean±SEM.
Table 2.
Baseline Artery Diameter.
| Premenopause | Perimenopause | Postmenopause | |||
|---|---|---|---|---|---|
| High-fit (n=11) | Low-fit (n=7) | High-fit (n=12) | Low-fit (n=8) | High-fit (n=13) | |
| Average pre-exercise baseline diameter (mm) | 3.4 ± 0.1 | 3.2 ± 0.1 | 3.3 ± 0.1 | 3.3 ± 0.1 | 3.4 ± 0.1 |
| Average post-exercise baseline diameter (mm) | 3.4 ± 0.1 | 3.2 ± 0.1 | 3.3 ± 0.1 | 3.3 ± 0.1 | 3.4 ± 0.1 |
Baseline artery diameter did not differ between groups before or after exercise. Differences within groups were assessed using paired t-tests and between groups using ANOVAs or an equivalent non-parametric test. Data are presented as mean±SEM.
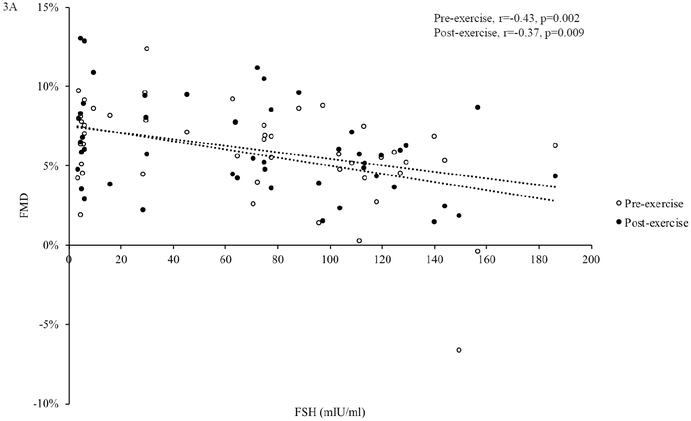
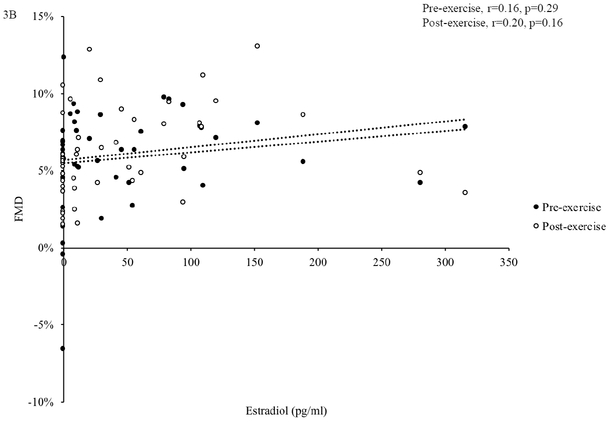
In all participants combined, pre- and post-exercise FMD were negatively related to age (pre-exercise: r=−0.33, p=0.020; post-exercise: age: r=−0.45, p=0.001) and FSH (pre-exercise: r=−0.43 p=0.002; post-exercise, r=−0.37, p=0.009, Figure 3A), but not estradiol (pre-exercise: r=0.16, p=0.29; post-exercise: r=0.20, p=0.16, Figure 3B).
Figure 3. Associations between Sex Hormones and FMD.
In all participants (n=51), follicle stimulating hormone (FSH) was negatively related to flow-mediated dilation (FMD) before and after acute exercise (A). Estradiol was not related to FMD before or after acute exercise (B). Associations were assessed using Pearson correlations.
Discussion
This study aimed to determine whether there was a decline in FMD with menopause in habitually active women with high cardiorespiratory fitness and to evaluate the influence of cardiorespiratory fitness on the FMD response to acute exercise in peri- and postmenopausal women. The findings were: 1) there was a decrease in FMD with progressive menopausal stage in highly-fit women, 2) high cardiorespiratory fitness was not related to higher FMD in healthy peri- or postmenopausal women, 3) cardiorespiratory fitness affected the FMD response to acute exercise in perimenopausal women, and 4) follicle stimulating hormone (FSH), but not estradiol was negatively related to FMD. These data suggest that there is reduced endothelial function with progressive menopausal stage and that this reduction is present in women with high cardiorespiratory fitness.
Moreau et al., reported a progressive decline in FMD with advancing menopausal stage15, while cross-sectional analyses demonstrated lower FMD in postmenopausal compared to premenopausal women12–14. Importantly, these studies generally evaluated women with low levels of cardiorespiratory fitness. Given the evidence supporting the protective influence of habitual physical activity and high fitness on vascular aging in men16,23,24, we hypothesized that highly fit women would not experience a reduction in FMD based on menopausal stage. Contrary to our hypothesis, we found that highly-fit postmenopausal women had lower function than highly-fit premenopausal women, suggesting that fitness does not attenuate the decline in FMD with menopause.
Regular endurance exercise and higher cardiorespiratory fitness are associated with optimal endothelial function; however, in midlife and older adults, this relation may differ based on sex. Pierce and colleagues demonstrated that active 55–79 year-old men had greater FMD than their sedentary counterparts, whereas postmenopausal active women did not. They also demonstrated a lack of improvement in FMD with 8 weeks of exercise training in sedentary postmenopausal women, while men improved FMD significantly with training16. The sex difference in the association between FMD and cardiorespiratory fitness in healthy individuals may be due to changes that occur with menopause. Findings from studies evaluating the FMD response to exercise training in postmenopausal women are equivocal. Some studies have reported improvements in FMD with training25,26, while others have shown no benefit of training on FMD16,27,28. Our recent review of this literature suggests that improvements in FMD due to exercise training may only occur in those with greater CVD risk burden29. In healthy women with low CVD risk, FMD appears to be more influenced by menopausal stage. The present analysis supports this hypothesis, as we show a decline in FMD with menopause in healthy women, despite high levels of cardiorespiratory fitness.
Our results also show that high fit peri- and postmenopausal women had lower FMD than low fit women. Most notably, average FMD in high-fit postmenopausal women was approximately 4.2%, which would indicate clinical endothelial dysfunction30. These low FMD values are in line with what others have reported in highly-fit postmenopausal women14. Interestingly, we found no association between FMD and VO2peak, suggesting that factors other than cardiorespiratory fitness are likely influencing FMD in these women. One explanation for the low FMD values we report is that remodeling of the vasculature has occurred in high-fit women. There is evidence to suggest that short term exercise training improves FMD; however, over time and with continued training, remodeling of the vasculature occurs, leading to a larger lumen:wall ratio and a lowering of FMD compared to after initial training began31,32. These studies were primarily conducted in young healthy men, therefore it has yet to be determined whether habitual exercise training creates the same adaptations in midlife women. Another possibility is that high-fit women are relying on vasodilatory factors other than nitric oxide, which FMD would not detect. The reliance on non-nitric oxide vasodilatory factors (e.g. endothelial derived hyperpolarizing factors) appears to increase when nitric oxide-mediated vasodilation is reduced33,34. Therefore, it is possible that we are seeing a shift in vasodilatory capacity that is less reliant on nitric oxide in high-fit women, leading to an overall lower FMD response. Based on these possibilities, a lower FMD response may not indicate true endothelial dysfunction; however, this requires future studies and investigation.
The lack of benefit of fitness on FMD through the menopausal transition may indicate that the effects of a changing hormonal environment outweigh the effects of habitual exercise. Estrogen has well-established effects on endothelial cells35,36. Moreau et al. reported that postmenopausal women did not improve FMD with exercise training unless they were supplemented with estradiol37. Interestingly, we found no association between FMD and estradiol, but a significant inverse relation between FSH and FMD. Follicle stimulating hormone levels rise during perimenopause and remain elevated in postmenopause19. Emerging evidence suggests that FSH may have an effect on CVD risk that is independent of the effects of changing estrogen levels38,39. The relation between FSH and endothelial function is relatively unexplored; however, women who had a lower increase in FSH through the menopause transition had a lower risk of atherosclerosis development compared to those with a moderate or high rise in FSH40. Endothelial dysfunction occurs early in the process of atherosclerosis, therefore, our data showing lower FMD with higher FSH is consistent with a potential role of FSH in vascular health. Similarly, Moore et al., reported an inverse association between vascular conductance and FSH in women at different menopausal stages41 and Moreau et al., reported an inverse association between FSH and FMD15. Therefore, these data suggest that the increase in FSH with menopause may negatively influence endothelial function. Given that postmenopausal women are exposed to chronically high levels of FSH, this may represent an important area of investigation for future mechanistic studies aimed at reducing CVD risk with menopause.
Assessment of the FMD response to acute exercise provides insight into the endothelial responsiveness to a cardiovascular challenge and may reveal differences in endothelial function that are not otherwise apparent42,43. We have previously reported different endothelial responses to acute moderate intensity treadmill exercise in healthy women. We showed no difference in FMD in low-fit peri- vs. postmenopausal women before exercise; however, perimenopausal women showed a trend for higher FMD after acute exercise17. In the current analysis, we also found a divergent response to acute exercise in perimenopausal women. We show that low-fit perimenopausal women had higher FMD compared with high-fit women following exercise. To our knowledge, this is the first evaluation of the effect of fitness on FMD responses to acute exercise in peri- and postmenopausal women.
The divergent response to acute exercise between low- and high-fit women may be driven by habituation to the exercise stimulus in high-fit women. This hypothesis is supported by the lack of FMD response to acute exercise in the high-fit premenopausal, perimenopausal, and postmenopausal groups. Interestingly, neither the high- nor the low fit postmenopausal group responded to acute exercise. The lack of responsiveness to acute exercise in postmenopausal women has been shown by at least one other group. Yoo et al., reported no FMD response to acute low, moderate or high intensity exercise in healthy postmenopausal women44. Together with our data, this suggests that postmenopausal status, regardless of cardiorespiratory fitness level, is associated with a lack of FMD responsiveness to acute exercise.
Limitations
While this study provides novel insight into differences in endothelial function based on menopausal status and cardiorespiratory fitness, it has some limitations. This study was a cross-sectional analysis; therefore, causation cannot be inferred from the results. Future work evaluating these outcomes longitudinally is warranted. Participants in this study were selected to have few risk factors for cardiovascular disease, to allow us to better evaluate the independent effects of menopausal status and cardiorespiratory fitness on FMD, while limiting potential confounding factors. While this may be considered a strength of the study, results may not be generalizable to a more diverse population. Given that menopause and aging are strongly connected, it is possible that some of the differences that we observed between groups were due to differences in age. In the current study, the range of ages across groups was as narrow as possible and there was only a weak relation between FMD and age. There was also no difference in age between high vs. low fit perimenopausal and high vs. low-fit postmenopausal women. Based on this, we do not believe that the differences we report are due to age. Finally, it is worth noting that sample sizes in this study were relatively small, therefore future research is needed to corroborate these findings.
Conclusions
Data from this study shows that there is a reduction in FMD with menopause in highly fit women. We also found that high cardiorespiratory fitness was not associated with better endothelial function in peri- or in postmenopausal women. Finally, we report that lower FMD was associated with higher circulating FSH. Our results suggest that factors other than cardiorespiratory fitness could be important targets for improving CVD risk in aging women. It also highlights the need for further research to better understand changes in endothelial function and the role of cardiorespiratory fitness in a menopausal population.
Acknowledgments:
We would like to acknowledge members of the Molecular and Cardiovascular Physiology Laboratory and study participants for their contributions to this project.
Financial Support: American College of Sports Medicine Foundation Doctoral Student Research Grant (Serviente), University of Massachusetts Amherst Faculty Research Grant (Witkowski), and National Institute on Aging Grant T32 AG049676 to The Pennsylvania State University.
Footnotes
Conflict of Interest Disclosure: None
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics−−2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54(25):2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321(10):641–646. [DOI] [PubMed] [Google Scholar]
- 4.El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Endogenous sex hormones impact the progression of subclinical atherosclerosis in women during the menopausal transition. Atherosclerosis. 2012;225(1):180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause. 2013;20(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambrinoudaki I, Armeni E, Georgiopoulos G, et al. Subclinical atherosclerosis in menopausal women with low to medium calculated cardiovascular risk. Int J Cardiol. 2013;164(1):70–76. [DOI] [PubMed] [Google Scholar]
- 7.Maturana MA, Franz RF, Metzdorf M, da Silva TR, Spritzer PM. Subclinical cardiovascular disease in postmenopausal women with low/medium cardiovascular risk by the Framingham risk score. Maturitas. 2015;81(2):311–316. [DOI] [PubMed] [Google Scholar]
- 8.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26(5):1235–1241. [DOI] [PubMed] [Google Scholar]
- 9.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol. 2008;51(10):997–1002. [DOI] [PubMed] [Google Scholar]
- 10.Taddei S, Virdis A, Ghiadoni L, et al. Menopause is associated with endothelial dysfunction in women. Hypertension. 1996;28(4):576–582. [DOI] [PubMed] [Google Scholar]
- 11.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24(2):471–476. [DOI] [PubMed] [Google Scholar]
- 12.Harvey P, Picton P, Su W, Morris B, Notarius C, Floras J. Exercise as an alternative to oral estrogen for amelioration of endothelial dysfunction in postmenopausal women. Am Heart J. 2005;149(2):291–297. [DOI] [PubMed] [Google Scholar]
- 13.Harvey P, Morris B, Kubo T, et al. Hemodynamic after-effects of acute dynamic exercise in sedentary normotensive postmenopausal women. J Hypertens. 2005;23(2):285–292. [DOI] [PubMed] [Google Scholar]
- 14.Santos-Parker JR, Strahler TR, Vorwald VM, Pierce GL, Seals DR. Habitual aerobic exercise does not protect against micro- or macrovascular endothelial dysfunction in healthy estrogen-deficient postmenopausal women. J Appl Physiol (1985). 2017;122(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab. 2012;97(12):4692–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce G, Eskurza I, Walker A, Fay T, Seals D. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci. 2011;120:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serviente C, Troy LM, de Jonge M, Shill DD, Jenkins NT, Witkowski S. Endothelial and inflammatory responses to acute exercise in perimenopausal and late postmenopausal women. Am J Physiol Regul Integr Comp Physiol. 2016;311(5):R841–R850. [DOI] [PubMed] [Google Scholar]
- 18.National Institutes of Health, National Heart, Lung, and Blood Institute. ATP III guidelines at-a-glance quick desk reference. Washington, DC: US Department of Health and Human Services; 2001. [Google Scholar]
- 19.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop 10: addressing the unfinished agenda of staging reproductive aging. Climacteric. 2012;15(2):105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson WR, Gordon NF, Pescatello LS. ACSM’s guidelines for exercise testing and prescription. Hubsta Ltd; 2009. [DOI] [PubMed] [Google Scholar]
- 21.GovTrack.us. S. 2748—110th Congress (2008): Physical Activity Guidelines for Americans Act of 2008,.
- 22.Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300(1):H2–H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seals DR. Edward F. Adolph Distinguished Lecture: The remarkable anti-aging effects of aerobic exercise on systemic arteries. J Appl Physiol. 2014;117(5):425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow‐mediated dilatation with sedentary and physically active human ageing. J Physiol (Lond). 2004;556(1):315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol. 2009;297(3):H1109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swift DL, Johannsen NM, Tudor-Locke C, et al. Exercise training and habitual physical activity: a randomized controlled trial. Am J Prev Med. 2012;43(6):629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klonizakis M, Moss J, Gilbert S, Broom D, Foster J, Tew GA. Low-volume high-intensity interval training rapidly improves cardiopulmonary function in postmenopausal women. Menopause. 2014;21(10):1099–1105. [DOI] [PubMed] [Google Scholar]
- 28.Casey DP, Pierce GL, Howe KS, Mering MC, Braith RW. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur J Appl Physiol. 2007;100(4):403–408. [DOI] [PubMed] [Google Scholar]
- 29.Witkowski S, Serviente C. Endothelial dysfunction and menopause: is exercise an effective countermeasure? Climacteric. 2018:1–9. [DOI] [PubMed] [Google Scholar]
- 30.Swift DL, Weltman JY, Patrie JT, et al. Predictors of Improvement in Endothelial Function after Exercise Training in a Diverse Sample of Postmenopausal Women. J Womens Health. 2013;23(3):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tinken TM, Thijssen DH, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol (Lond). 2008;586(20):5003–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev. 2017;97(2):495–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozkor MA, Quyyumi AA. Endothelium-derived hyperpolarizing factor and vascular function. Cardiol Res Pract. 2011;2011:156146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schinzari F, Tesauro M, Cardillo C. Vascular hyperpolarization in human physiology and cardiovascular risk conditions and disease. Acta Physiologica. 2017;219(1):124–137. [DOI] [PubMed] [Google Scholar]
- 35.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60(2):210–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Usselman CW, Stachenfeld NS, Bender JR. The molecular actions of oestrogen in the regulation of vascular health. Exp Physiol. 2016;101(3):356–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function With endurance exercise in postmenopausal women. J Clin Endocrinol Metab. 2013;98(11):4507–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang N, Shao H, Chen Y, et al. Follicle-Stimulating Hormone, Its Association with Cardiometabolic Risk Factors, and 10-Year Risk of Cardiovascular Disease in Postmenopausal Women. J Am Heart Assoc. 2017;6(9): 10.1161/JAHA.117.005918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertone-Johnson ER, Virtanen JK, Nurmi T, et al. Follicle-Stimulating Hormone Levels and Subclinical Atherosclerosis in Older Postmenopausal Women. Am J Epidemiol. 2017;187(1):16–26. [DOI] [PubMed] [Google Scholar]
- 40.El Khoudary SR, Santoro N, Chen HY, et al. Trajectories of estradiol and follicle-stimulating hormone over the menopause transition and early markers of atherosclerosis after menopause. Eur J Prev Cardiol. 2016;23(7):694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore DJ, Gonzales JU, Tucker SH, Elavsky S, Proctor DN. Exercise-induced vasodilation is associated with menopause stage in healthy middle-aged women. Appl Physiol Nutr Metab. 2012;37(3):418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dawson EA, Green DJ, Cable NT, Thijssen DH. Effects of acute exercise on flow-mediated dilatation in healthy humans. J Appl Physiol (1985). 2013;115(11):1589–1598. [DOI] [PubMed] [Google Scholar]
- 43.Padilla J, Harris RA, Wallace JP. Can the measurement of brachial artery flow-mediated dilation be applied to the acute exercise model. Cardiovasc Ultrasound. 2007;5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo J, Hwang M, Kim H, Hwang E, Handberg EM, Christou D. Effect of High-intensity Interval Training on Endothelial Function in Older Postmenopausal Women: a Randomized Controlled Trial. FASEB J. 2016;30(1 Supplement): 763.14–763.14. [Google Scholar]