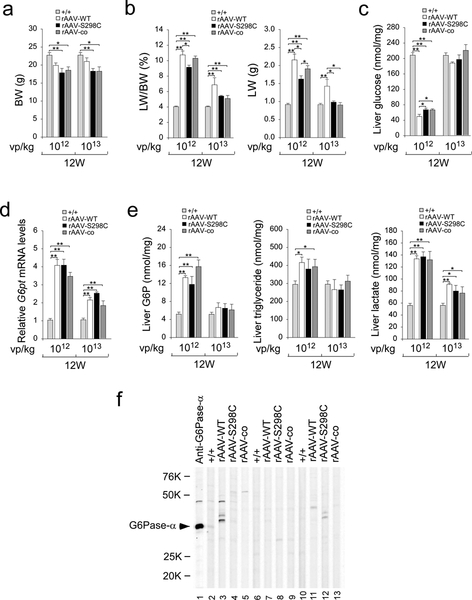
Fig. 4.
Phenotypic analyses of rAAV-treated G6pc−/− mice at age 12 weeks. G6pc−/− mice were treated at age 2 weeks with either 1012 vp/kg or 1013 vp/kg of rAAV-WT, rAAV-S298C, or rAAV-co, and analyzed at age 12 weeks. The data were obtained from WT (+/+, n = 12) and G6pc−/− mice treated with either 1012 vp/kg (n = 6 per vector) or 1013 vp/kg (n = 6 per vector) of rAAV-WT, rAAV-S298C, or rAAV-co. (a) BW. (b) LW/BW ratios and LW. (c) Hepatic glucose levels. (d) Relative G6pt mRNA levels. (e) Hepatic levels of G6P, triglyceride, and lactate. (f) Western-blot analysis of serum antibodies against human G6Pase-α. The serum samples were obtained from 12 week-old G6pc−/− mice treated at age 2 weeks with 1013 vp/kg of rAAV-WT, rAAV-S298C, or rAAV-co vector. Lanes 1: anti-hG6Pase-α antiserum; lanes 2, 6, 10: serum samples (1: 50 dilution) from WT mice; lanes 3, 7, 11: serum samples (1: 50 dilution) from rAAV-WT-treated G6pc−/− mice; lanes 4, 8, 12: serum samples (1: 50 dilution) from rAAV-S298C-treated G6pc−/− mice; and lanes 5, 9, 13: serum samples (1: 50 dilution) from rAAV-co-treated G6pc−/− mice. Data represent the mean ± SEM. *p < 0.05, **p < 0.005.