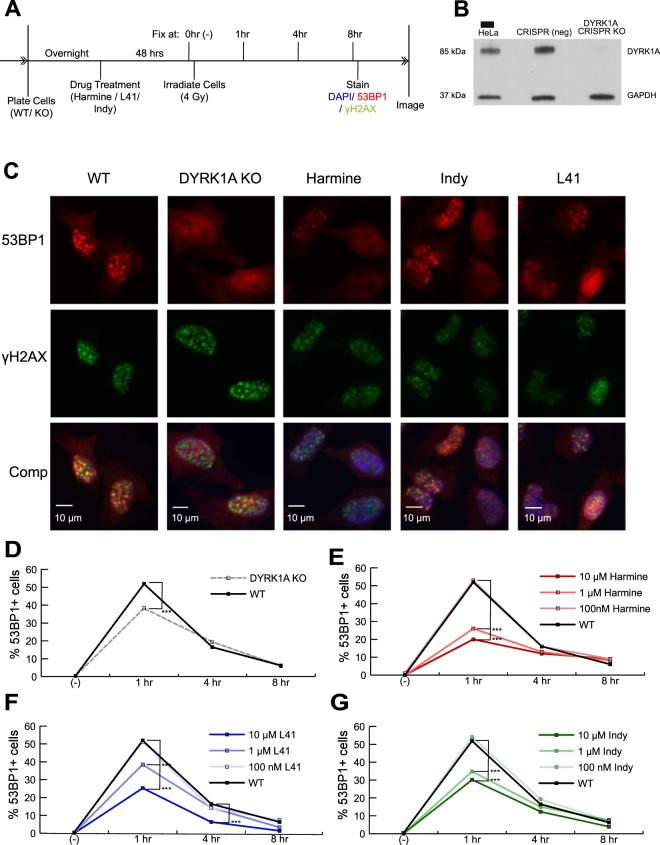
Figure 3.
DYRK1A expression and kinase activity are necessary for efficient 53BP1 recruitment to DNA double-strand break sites. (A) Experimental timeline for 53BP1 foci quantitation in response to DYRK1A manipulation and IR. Cells were treated with drug 48 hours prior to treatment with 4 Gy of IR. Cells were fixed immediately before (0 hrs) or 1, 4 and 8 hrs following IR treatment. (B) Western blot confirmation of DYRK1A CRISPR knockout in HeLa cells. DYRK1A and GAPDH loading control bands were cropped from portions of the same blot (Full molecular weight range seen in Suppl. Fig. S3E). (C) Representative immunofluorescent images of γH2AX and 53BP1 staining in fixed HeLa cells. Cells were fixed in 96-well plates. Four frames per well were imaged for each well. Four wells per condition per time point were plated and quantified using the Focinator R package79. N ≈ 400–1000 cells per condition. Blue: Hoechst; Green: γH2AX; Red: 53BP1. 53BP1 + cells ≥ 10 foci/cell. (53BP1 noise cut off: 15; γH2AX noise cut off 20). (D) DYRK1A KO HeLa cells: Proportion of 53BP1 + cells over time following 4 Gy of IR (***p < 0.001). (E–G) WT HeLa cells were treated with Harmine, L41 or INDY for 48 hours prior to irradiation: Proportion of 53BP1 + cells over time following 4 Gy of IR (***p < 0.001).