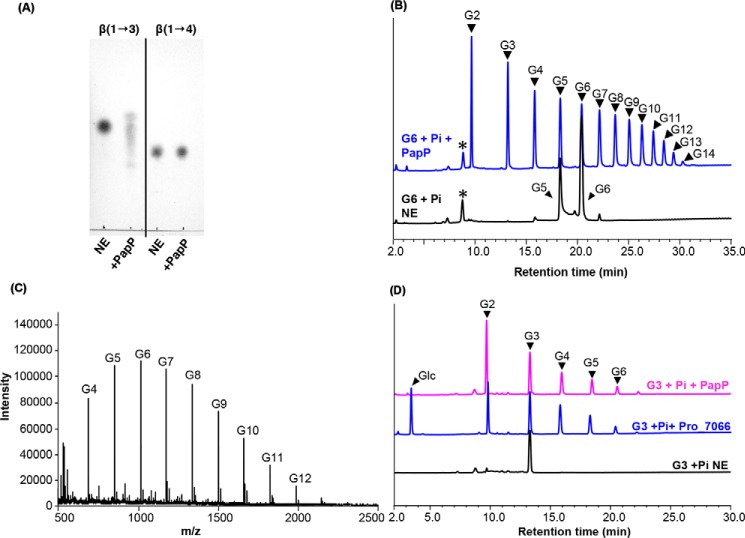
Figure 4.
Characterization of PapP and comparison of PapP substrate chain length preference with that of GH149 enzyme. A, TLC analysis of the phosphorolysis reaction carried out by PapP in the presence of 20 mm laminaritriose (β-(1→3)-linkage) or cellotriose (β-(1→4)-linkage). B, HPAEC-PAD analysis of the phosphorolysis reaction of 20 mm G6 carried out by PapP in the presence of 10 mm Pi. G5 was detected as a contaminant in the starting material. C, MALDI-TOF analysis of the G6 + Pi + PapP reaction in B. G2–G14, DP of the β-(1→3)-gluco-oligosaccharide products. NE, no enzyme control. All assays were performed in 100 mm HEPES, pH 7.0, at 30 °C for 1 h. *, unknown peaks. D, comparison between the phosphorolysis carried out by either PapP (magenta) or Pro_7066 from GH149 family (blue) in the presence of 20 mm G3 and 10 mm Pi as substrates.