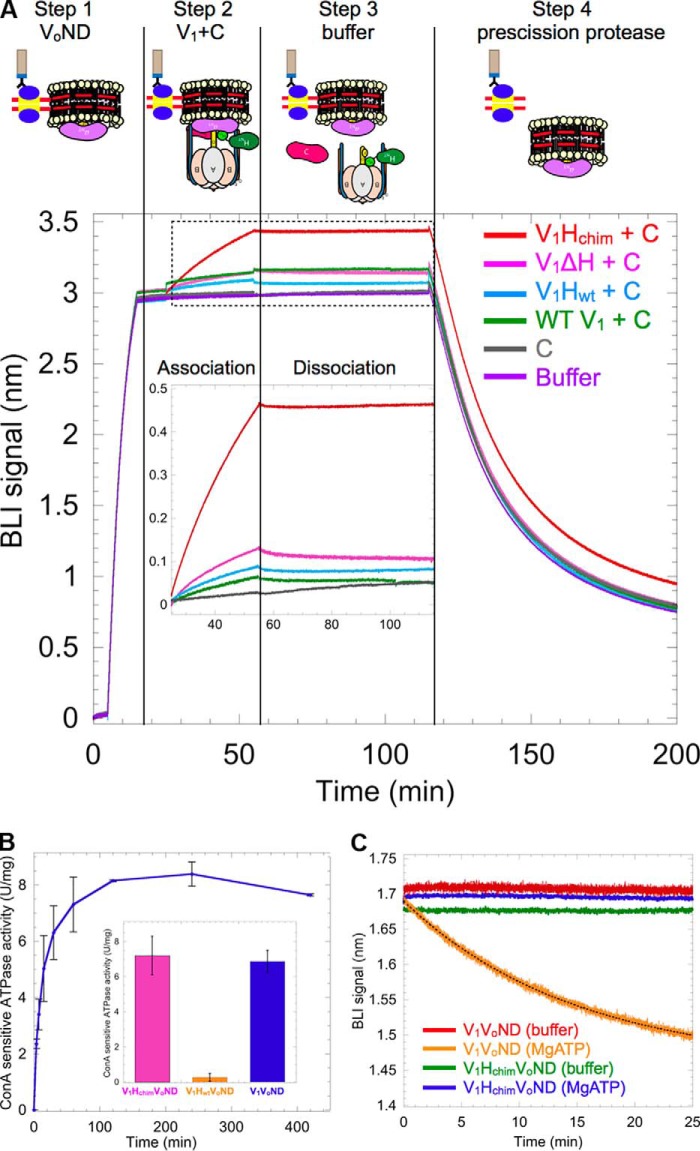
Figure 3.
V1Hchim and C associate with VoND to form coupled V1Vo-ATPase. A, VoND was immobilized on streptavidin-coated BLI sensors via biotinylated MSP (step 1). Sensors were then dipped into 0.4 μm of V1 mutants in presence of 1 μm C (association; step 2) followed by buffer (dissociation; step 3). Association with VoND was most efficient with V1Hchim (red trace). Sensors were then dipped in PreScission protease to verify that the BLI signal was not due to nonspecific binding (step 4). Inset shows an enlarged view of the association and dissociation steps. B, equimolar amounts of V1Hchim and VoND, and a 2-fold molar excess of C subunit were incubated at 22 °C, and the ConA-sensitive MgATPase activity was measured as a function of time. Each point represents the mean ± S.E. of two separate reconstitutions from two individual purifications. Inset, specific MgATPase activities of reconstituted V1HchimVoND and V1HwtVoND (± S.E. from two independent purifications) compared with purified V1VoND (29). C, following association of the V1HchimVoND complex, sensors were dipped in wells containing buffer (green) or buffer + 1 mm MgATP (blue) for dissociation rate measurement. The dissociation phase of WT V1VoND in buffer (red) and buffer +1 mm MgATP (orange) is included for comparison (data from Ref. 29).