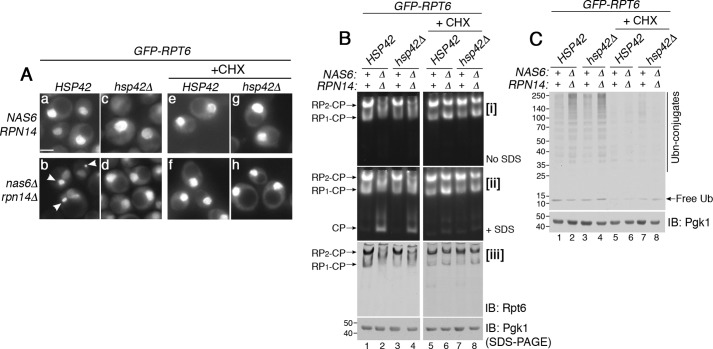
Figure 4.
Rpt subunits proceed to proteasome assembly when the free Rpt pool is limited. A, sequestration of Rpt subunits can be blocked upon deletion of HSP42. Live-cell images using epi-fluorescence microscopy were obtained upon heat stress at 37 °C for 1 h (panels a–d). The same experiments were also conducted in the presence of cycloheximide (panels e–h, CHX, 150 μg/ml). Arrowheads in panel b indicate GFP–Rpt6 puncta. Scale bar, 5 μm for all panels. B, Rpt subunits incorporate into the proteasome holoenzyme, not simply when their sequestration is blocked but when their continued synthesis is blocked (see text for details). Indicated yeast strains were grown in the absence or presence of cycloheximide (150 μg/ml) at 37 °C for 2 h. Proteasome assembly and activities were assessed by subjecting whole-cell lysates (60 μg) to native PAGE and in-gel peptidase assays without or with 0.02% SDS in panels i and ii, respectively. Proteasome holoenzyme levels were examined by immunoblotting (IB) with anti-Rpt6 antibody (panel iii). Pgk1 is a loading control. The Pgk1 blot in lanes 1–4 and 5–8 derives from two different gels, which were processed the same in parallel during immunoblotting and signal detection. C, cellular ubiquitinated proteins are degraded upon efficient assembly of the proteasome holoenzyme. Whole-cell lysates (20 μg) were obtained from yeast cells that were treated as in B. The lysates were subjected to 10% BisTris SDS-PAGE and immunoblotting with an antibody to ubiquitin. Pgk1 is a loading control.