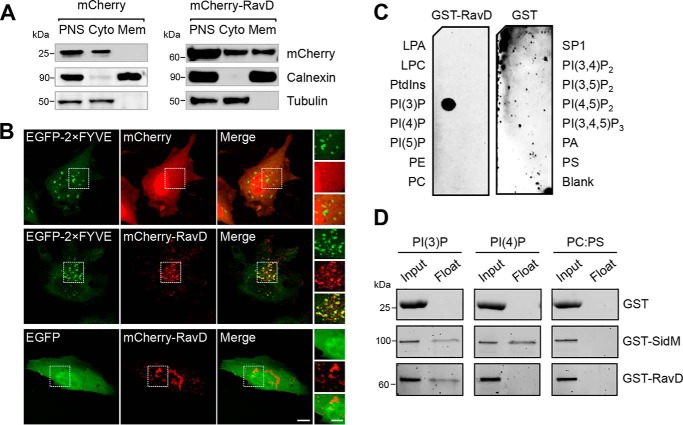
Figure 1.
RavD localizes to membrane compartments positive for PI(3)P and binds PI(3)P in vitro. A, RavD is cytosolic and membrane-bound. HEK293T cells producing mCherry-RavD or mCherry alone were homogenized, and the post-nuclear supernatant (PNS) was subjected to cellular fractionation. An anti-mCherry antibody was used to detect the presence of RavD. Antibodies against tubulin and calnexin were used to mark the cytosolic (Cyto) and membrane (Mem) fractions, respectively. B, confocal images of HeLa cells transiently co-transfected with plasmids encoding either EGFP-2×FYVE or EGFP and mCherry-RavD or mCherry. Scale bars = 10 μm (insets, 2 μm). C, protein–lipid overlay assay showing that GST-RavD specifically recognizes PI(3)P. A nitrocellulose membrane prespotted with 100 pmol of each indicated lipid was incubated with purified GST-RavD. RavD retained on the membrane was detected by incubation with anti-GST and an HRP-conjugated secondary anti-rabbit antibody. LPA, lysophosphatidic acid; LPC, lysophosphocholine; PtdIns, phosphatidylinositol; PE, phosphatidylethanolamine; PC, phosphatidylcholine; SIP, sphingosine-1-phosphate; PI, phosphatidylinositol; P, phosphate; P2, biphosphate; P3, triphosphate; PA, phosphatidic acid; PS, phosphatidylserine. D, liposomes containing the indicated lipids were incubated with GST-RavD, GST-SidM, or GST alone and subjected to ultracentrifugation to separate bound from unbound protein. Input and float samples were separated on 4–15% TGX stain–free SDS-PAGE gels. Results are representatives of at least two independent experiments with similar outcomes.