Figure 1.
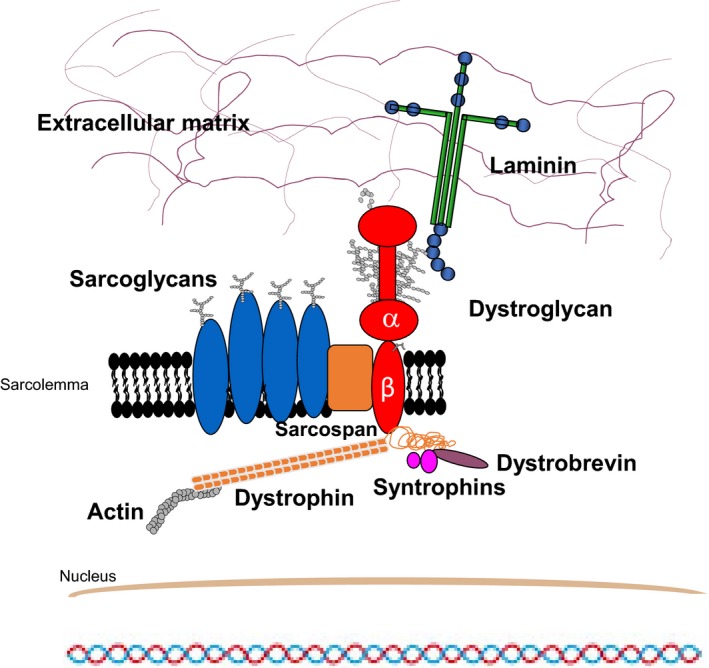
Schematic representation of the dystrophin‐glycoprotein complex (DGC) in skeletal muscle. The two dystroglycan subunits interact non‐covalently to form a bridge between the extracellular matrix and the actin cytoskeleton. α‐DG and β‐DG are non‐covalently connected and they also interact with numerous other proteins. The cytosolic domain of β‐DG is anchored to actin through the interaction with dystrophin and β‐DG also constitutes a scaffold for proteins involved in signal transduction such as Gbr2 and ERK. α‐DG is a so‐called peripheral membrane protein that interacts with the ectodomain of β‐DG on the extracellular side of the plasma membrane. α‐DG acts as a receptor for extracellular matrix proteins such as laminins (reported in the scheme), perlecan, neurexins and agrin among others
