Figure EV4. Clamp movements observed by alternative fluorophore positions.
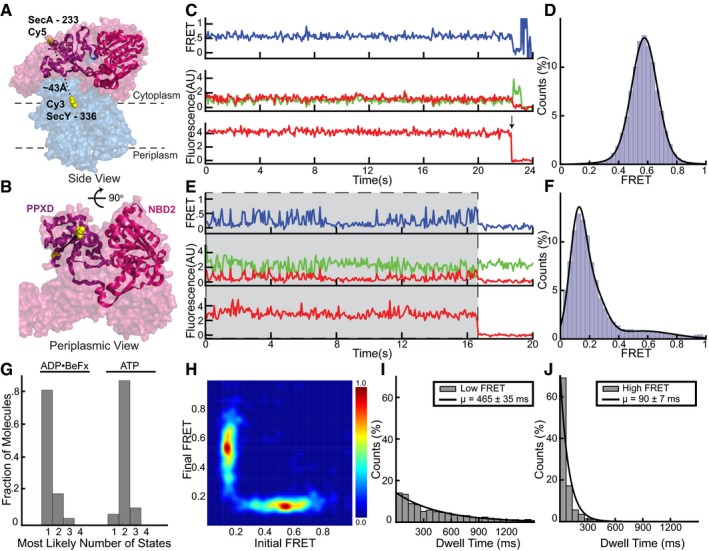
- Cy5 and Cy3 fluorophores were introduced into the clamp of SecA (PDB 3DIN; red space filling model) at position 233 and into the C‐terminal half of SecY (blue) at position 336, respectively. The PPXD and NBD2 making up the clamp are shown as a violet and magenta ribbon models, respectively.
- Rotated view of (A) with SecY masked except for the labeled residue 336.
- Representative traces obtained with ADP•BeFx. The upper FRET trace was calculated from the middle traces obtained by exciting the donor fluorophore and measuring both donor (green) and acceptor (red) fluorescence. The lowest trace was obtained by exciting the acceptor fluorophore directly. The arrow indicates a bleaching event.
- Distribution of FRET values determined from 163 traces as in (B), fit with a Gaussian model (black curve).
- As in (C), but in the presence of ATP. Periods in which a fluorescently labeled SecA molecule is bound are indicated by gray shading.
- As in (D), but with ATP (178 traces).
- Traces obtained in the presence of different nucleotides were used to determine the number of states best fit by the Markov model.
- Transition density plot of idealized ATP FRET states obtained in (F).
- The distribution of dwell times of the low FRET states observed in ATP was fit with an exponential (1,550 low FRET states). The inset shows average dwell time and error, defined as the standard error based on the number of traces.
- As in (I), but with high FRET (1,778 high FRET states).
Source data are available online for this figure.
