Figure 1. ATG16L1 membrane targeting activity is retained in the absence of ATG5 or WIPI2.
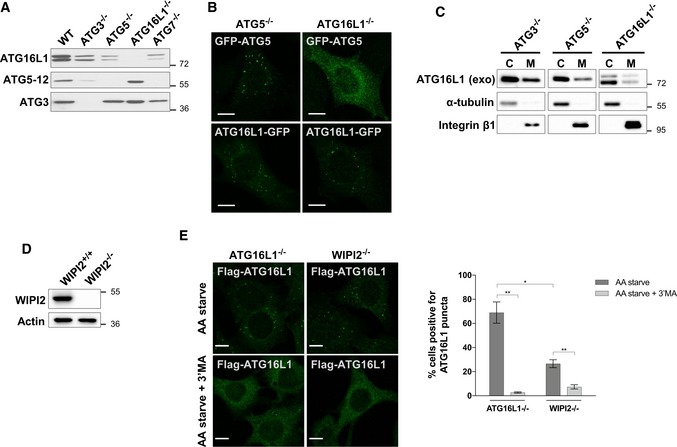
- Analyses of protein expression in lysates of various cell lines by Western blotting against the indicated antibodies.
- Fluorescence analyses of GFP‐ATG5 or ATG16L1‐GFP stably expressed in ATG5−/− or ATG16L1−/−. Cells were amino acid starved for 2 h prior to fixation and imaging of the GFP fluorescence. Scale bar: 10 μm.
- Assessment of ATG16L1 levels in the cytosolic (C) and membrane (M) fractions in lysates of the indicated cell lines using Western blot analyses and antibodies against ATG16L1. ATG16L1−/− and ATG5−/− stably expressed ATG16L1‐GFP while ATG3−/− stably expressed Flag‐S‐ATG16L1. Antibodies against α‐tubulin and integrin β1 were used as controls for fractionation. Exogenous (exo) ATG16L1 was detected using antibodies against ATG16L1.
- Lack of WIPI2 expression is confirmed by Western blot analyses in wild‐type MEFs (WIPI2+/+) and WIPI2−/− cells.
- Immunofluorescence analyses of ATG16L1−/− and WIPI2−/− stably expressing Flag‐S‐ATG16L1. Cells were amino acid starved (AA starve) for 2 h in the presence or absence of 3′MA (and additional pretreatment for 30 min) followed by fixation and immunostaining using antibodies against Flag tag to detect ATG16L1. Scale bar: 9 μm. Right panel represents quantification of three independent experiments and error bars depicting SEM values. *P ≤ 0.05, **P ≤ 0.01 (pairwise unpaired Student's t‐test).
