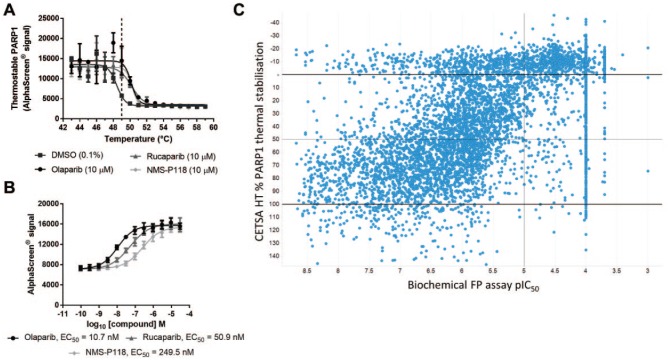
Figure 2.
A CETSA HT assay to screen for intracellular PARP1 target engagement. (A) MDA-MB-436 cells were treated for 1 h with DMSO, olaparib (10 µM), rucaparib (10 µM), or NMS-P118 (10 µM) before applying indicated heatshock, lysis, and quantification of thermostable PARP1 by AlphaScreen. Treatment of live cells with PARP inhibitors led to a thermal stabilization of PARP1. Data are the mean ± span of n = 2. (B) ITDRFCETSA experiments to rank intracellular PARP1 target engagement. MDA-MB-436 cells were treated with a concentration response of PARP inhibitors for 1 h prior to a 49 °C heatshock, lysis, and quantification of thermostable PARP1 by AlphaScreen. Thermal stabilization as a measure of target engagement allowed compounds to be ranked by apparent potency. Data are the mean ± standard deviation of n = 10. (C) Single-concentration CETSA HT screening of a library of PARP1 binders. The affinity (pIC50) of 6288 compounds to PARP1 protein was determined by concentration–response experiments using a biochemical FP assay (x axis). The same compounds were screened for CETSA HT thermal stabilization at 10 µM, plotted as percent PARP1 thermal stabilization relative to 100% olaparib stabilized (y axis).