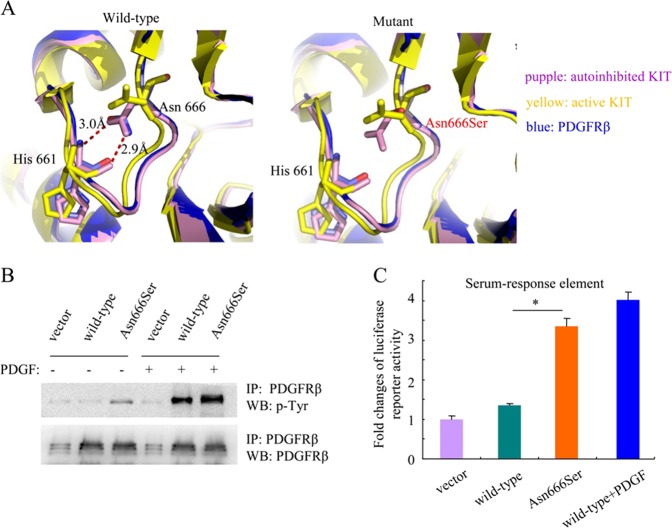
Fig. 2.
The PDGFRβ Asn666Ser mutant constitutively activated the receptor kinase activity. a A ribbon diagram shows the interaction between Asn666 and His661 in the PDGFRβ model (blue). The structure of the autoinhibited (purple) and active (yellow) forms of KIT kinase are shown for comparison. The p.Asn666Ser change would abolish the interaction linking Asn666 and His661. b Analysis of the phosphorylation and expression levels of the wild-type and mutated receptors by western blot. NIH3T3 cells stably expressing wild-type or mutant PDGFRβ were starved for 6 h and stimulated with PDGF-BB (20 ng/ml) for 15 min or left untreated before lysis. PDGFRβ was immunoprecipitated and analyzed by western blot experiments using an anti-phosphotyrosine antibody. c The activity of the PDGFRβ Asn666Ser mutant was analyzed in a luciferase reporter assay. NIH3T3 cells were transiently cotransfected with empty vector, wild-type or mutated PDGFRB receptors and a luciferase gene downstream of a SRE promoter. Four hours after transfection, cells were washed and treated or not with PDGF-BB (20 ng/ml) for 24 h. The histogram represents the fold changes in luciferase activity with SEM. Independent experiments were performed three times. *p < 0.05