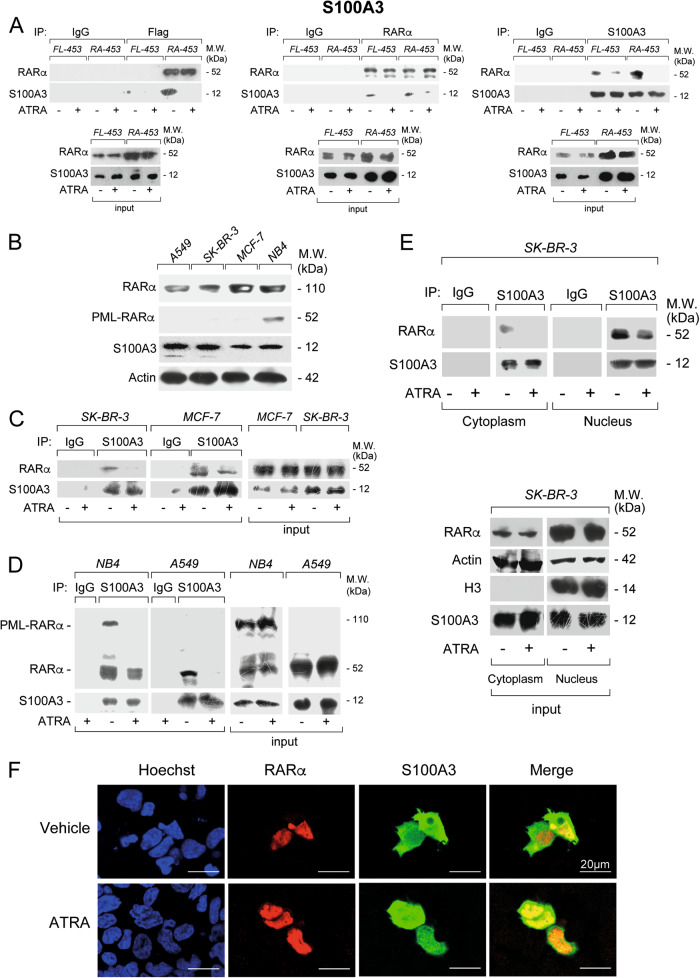
Fig. 1.
Interactions between S100A3 and RARα in breast cancer FL-453, RA-453, SK-BR-3, and MCF7 cells, lung cancer A549 cells as well as APL-derived NB4 cells. a FL-453 and RA-453 cells were treated with vehicle (DMSO) or ATRA (1 µM) for 1 h. At the end of the treatment, total cell extracts were immunoprecipitated with anti-FLAG mouse monoclonal antibodies (left), anti-RARα mouse monoclonal antibody (middle), and anti-S100A3 mouse monoclonal antibodies (right) or the corresponding non-specific immuno-globulins G (IgG) as negative controls. Following normalization for the content of RARα or S100A3 in the input, the various immunoprecipitates were subjected to western blot analysis with an anti-RARα rabbit polyclonal antibody or the anti-S100A3 antibody, as indicated. M.W. = molecular weights of the indicated proteins. Input = western blot analysis of the cell extracts before the indicated immunoprecipitation step. Each immunoprecipitation is representative of at least two independent experiments providing the same type of results. b Extracts from logarithmically growing breast cancer SK-BR-3 and MCF7 cells, lung cancer A549 cells as well as APL-derived NB4 cells were subjected to western blot analysis with antibodies targeting RARα and PML-RARα, S100A3, and β-actin. The molecular weights of the indicated proteins are shown on the right. SK-BR-3 and MCF7 (c) as well as NB4 and A549 (d) cells were treated with vehicle (DMSO) or ATRA (1 µM) for 1 h. At the end of the treatment, total cell extracts were immunoprecipitated with anti-S100A3 mouse monoclonal antibodies (IP: S100A3). The negative control for the immunoprecipitations is represented by the extracts challenged with non-specific immuno-globulins G (IP: IgG), as indicated. Following normalization for the content of S100A3 in the input, the immunoprecipitates were subjected to western blot analysis with anti-RARα and anti-S100A3 antibodies, as indicated. Input = western blot analyses of the cell extracts before the immunoprecipitation step. M.W. = molecular weights of the indicated proteins. e SK-BR-3 cells were treated with vehicle (DMSO) or ATRA (1 µM) for 1 h. At the end of the treatment, the nuclear (Nucleus) and the cytoplasmic (Cytoplasm) fractions of the cells were separated by centrifugation and subjected to immunoprecipitation with the anti-S100A3 antibody or the control IgG. As in (c), the immunoprecipitates were subjected to western blot analysis with anti-RARα and anti-S100A3 antibodies. Input = western blot analyses of the cell extracts before the immunoprecipitation step. The input data obtained with the nuclear marker, Histone H3 (H3), demonstrate efficient separation of the nuclear from the cytoplasmic fractions. Each immunoprecipitation is representative of at least two independent experiments providing the same type of results. f MDA-MB-453 cells were co-transfected with RARα and S100A3 expression plasmids. Twenty-four hours following transfection, cells were challenged with primary anti-RARα rabbit polyclonal antibodies mouse and anti-S100A3 monoclonal antibodies. Subsequently the cell slides were labeled with red-fluorescent anti-rabbit-Ig and green fluorescent anti-mouse-Ig secondary antibodies. Cell nuclei are shown in blue following staining with the Hoechst dye. Merging of the red- and green-fluorescence images is shown in the rightmost panels