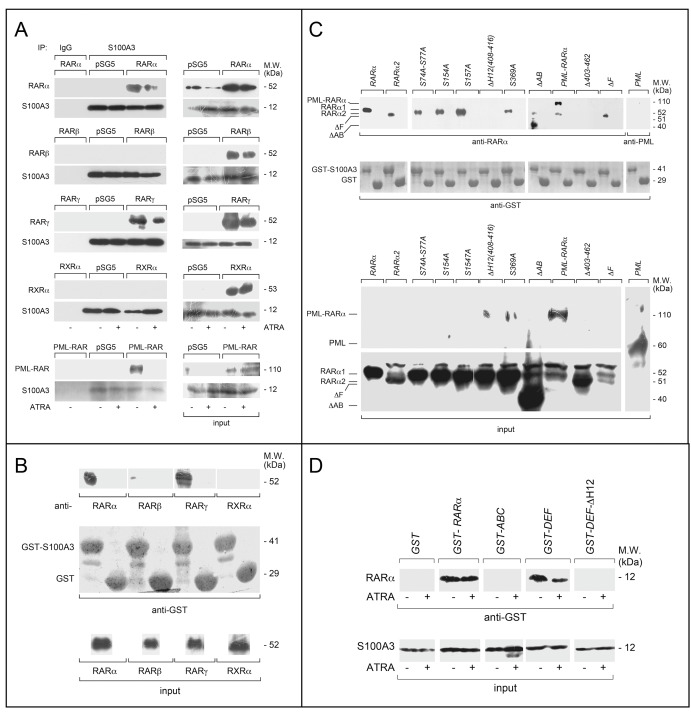
Fig. 2.
Specificity and structural determinants of RARα binding to S100A3. a COS-7 cells were co-transfected with equal amounts of RARα, PML-RARα, RARβ, RARγ or RXRα, and S100A3 expression plasmids, as indicated. The negative control for the experiments is represented by cells co-transfected with the void expression plasmid (pSG5). Twenty-four hours following transfection, cells were treated with vehicle (DMSO) or ATRA (1 µM) for 1 h. At the end of the treatment, total cell extracts were immunoprecipitated with anti-S100A3 mouse monoclonal antibodies (IP: S100A3). A further negative control for the immunoprecipitations is represented by the extracts of COS-7 cells co-transfected with pSG5 and the S100A3 expression plasmid which were challenged with non-specific immuno-globulins G (IP: IgG). Following normalization for the content of S100A3 in the input, the various immunoprecipitates were subjected to western blot analysis with anti-RARα, anti-RARβ, anti-RARγ, or anti-RXRα antibodies. All the blots were subsequently re-challenged with anti-S100A3 antibodies, as indicated. Input = western blot analysis of the cell extracts before the indicated immunoprecipitation step. Each immunoprecipitation is representative of at least two independent experiments providing the same type of results. b, c GST pull-down: the GST-tagged recombinant protein, GST-S100A3, and the GST negative control were used. The two recombinant proteins conjugated to Glutathione-Sepharose beads were incubated with extracts of COS-7 cells transfected with the pSG5 expression plasmids containing wild-type RARα, RARβ, RARγ, RXRα, RARα2, PML-RARα and the indicated RARα deletion-mutants and point-mutants. GST pull-down precipitates were blotted on nitro-cellulose filters, incubated with an anti-RARα, anti-RARβ, anti-RARγ, anti-RXRα antibodies (b) or anti-RARα antibodies only (c). Subsequently the filters were re-blotted with an anti-GST antibody, as indicated. Input: cell extracts (15 μg of protein) representing 10% of the total amount of protein were subjected to western blot analysis with the above anti-RARα antibody. d Far-western: COS-7 cells were transfected with the same S100A3 expression plasmid as in (a). Cell extracts were precipitated with sepharose beads conjugated with an anti-S100A3 monoclonal antibody. The immunoprecipitates were subjected to far-western analysis using the following GST-tagged RARα recombinant proteins: GST-RARα = full-length RARα; GST-ABC = RARα ABC regions; GST-DEF = RARα DEF regions; GST-DEFΔH12 = RARα1 DEF regions lacking the H12-helix. The blots were developed with an anti-GST antibody. Input: cell extracts (15 μg of protein) representing 10% of the total amount of protein used for the immunoprecipitations were subjected to western blot analysis with an anti-S100A3 antibody. Each line shows cropped lanes of the same gel, hence the results can be compared across the lanes, as they were obtained with the same exposure time. M.W. = molecular weights of the indicated proteins