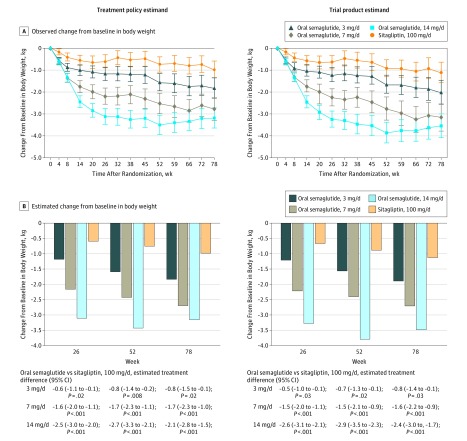
Figure 3. Body Weight–Related Efficacy End Points.
Data in panel A are observed mean change from baseline values (with 95% confidence intervals shown as error bars) for the “in-trial” period (ie, while participants remained in the trial regardless of trial product discontinuation or rescue medication use) and the “on-treatment without rescue observation” period (ie, while patients were receiving trial product without use of rescue medication), and data in panel B are estimated mean changes from baseline by the treatment policy estimand and the trial product estimand at weeks 26, 52 and 78. Unadjusted 2-sided P values are given for the test of no difference. Treatment policy estimand: analysis of covariance using data irrespective of discontinuation of trial product or initiation of rescue medication. Missing values were imputed by a pattern mixture model using multiple imputation. Pattern was defined by randomized trial product and treatment status (premature trial product discontinuation and/or initiation of rescue medication). Trial product estimand: mixed model for repeated measurements. Data collected after discontinuation of trial product or initiation of rescue medication were excluded.