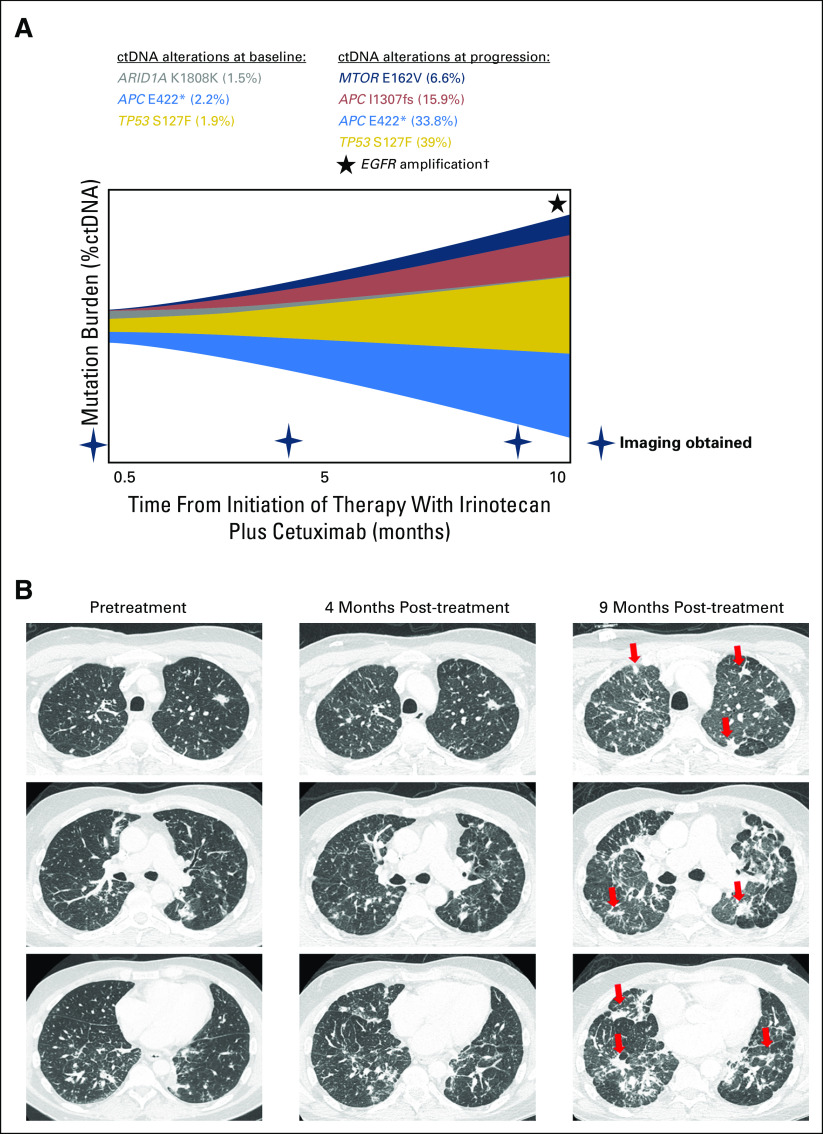
Fig 4.
Patients who had serial circulating tumor DNA (ctDNA) testing and were progressing on anti-EGFR–based therapy. A 49-year-old-man with metastatic adenocarcinoma of the rectum had a history of previous treatment with (1) capecitabine plus oxaliplatin and (2) fluorouracil plus irinotecan plus bevacizumab; (3) treatment on a clinical trial with anti-CD73 included fourth-line therapy with irinotecan plus cetuximab. (A) Patient’s baseline ctDNA at the start of therapy showing alterations (amount in percent). (B) The patient showed initial tumor regression, but at 9 months, the tumor progressed with new lung metastases and lymphangitic spread (red arrow). ctDNA among previously observed alterations increased approximately 20-fold (33.8% for APC E422*; 39% for TP53 S127F) along with emerging alterations, including MTOR E162V, APC I1307fs, and EGFR amplification. Among the ctDNA alterations observed in this patient, the following were characterized alterations: TP53 S127F, APC E422*, APC I1307fs, and EGFR amplification. MTOR E162V was a variant of uncertain significance; ARID1A K1808K was a synonymous substitution. (†) Only levels of ctDNA mutations were quantified using %ctDNA and represented; EGFR amplification was detected at progression but not quantified.