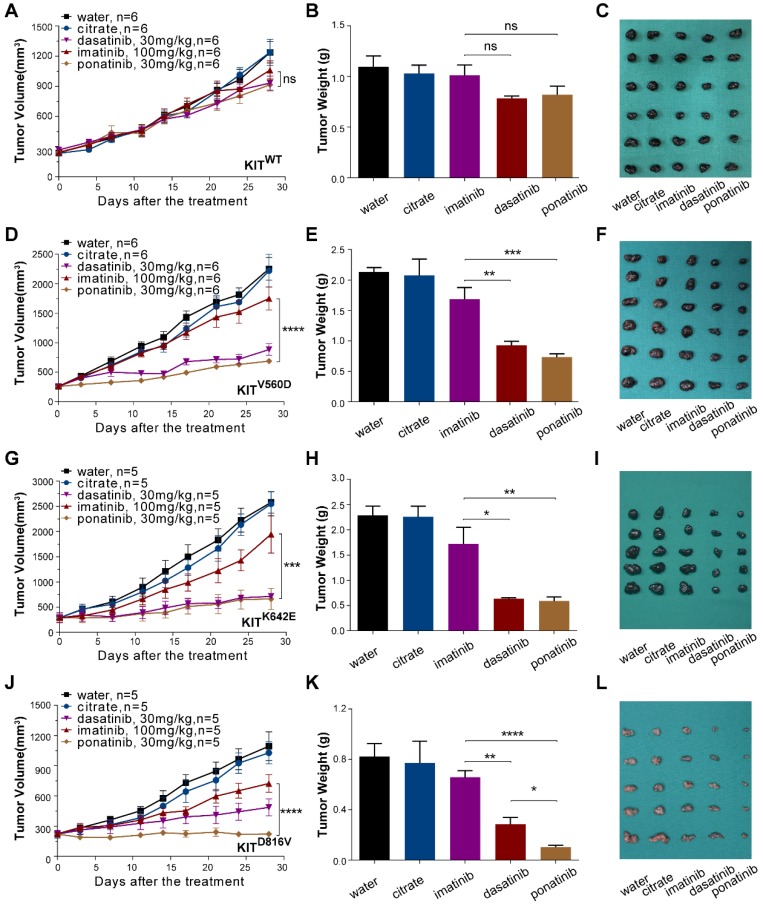
Figure 4.
In vivo drug efficacy evaluation in KITWT, KITV560D, KITK642E and KITD816V mutant PDX models. We administered imatinib (100 mg/kg), dasatinib (30 mg/kg) and ponatinib (30 mg/kg) to PDX mice daily for 28 days. Tumor volume and tumor weight were measured, and the results are summarized as the mean ± SEM (Student's t-test). Representative tumor images of these experiments (taken on day 28). (A-C) PDX-KITWT, treated with imatinib (TGI = 17.96%), dasatinib (TGI = 33.85%), and ponatinib (TGI= 33.26%), n = 6. (D-F) PDX with KITV560D mutation, treated with imatinib (TGI = 25.25%), dasatinib (TGI = 68.65%), and ponatinib (TGI = 78.33%), n = 6. (G-I) PDX with KITK642E mutation, treated with imatinib (TGI = 27.59%), dasatinib (TGI = 81.38%) and ponatinib (TGI = 83.66%), n = 5. (J-L) PDX with KITD816V mutation, treated with imatinib (TGI = 42.67%), dasatinib (TGI = 67.73%) and ponatinib (TGI = 99.95%), n = 5 *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant; n indicates the number of tumors per arm.