Abstract
Purpose
To determine the effects of αvβ3 integrin expression and activation on intraocular pressure (IOP).
Methods
Cre+/−β3flox/flox mice were treated with topical tamoxifen eye drops for 5 days to activate Cre and excise the β3 integrin gene from the anterior segment. IOP was measured weekly for 11 weeks using rebound tonometry. Mice were then killed and changes in expression of the β3 integrin subunit in Cre+/− β3flox/flox mice were determined using Western blotting analysis and immunofluorescence microscopy. To determine the effect of αvβ3 integrin activation on outflow facility, porcine organ culture anterior segments (POCAS) were perfused with the αvβ3 integrin-activating antibody AP5 or an isotype IgG control for 21 hours. The effect of αvβ3 integrin activation on IOP was measured over 7 days in C57BL/6J mice intracamerally infused with AP5, AP3, IgG, or PBS.
Results
Deletion of the β3 integrin subunit using the tamoxifen-inducible Cre-loxP system resulted in a decrease in expression of the β3 integrin subunit in the trabecular meshwork and ciliary muscle. Morphologically no gross changes in the anterior segment were detected. Deletion of the β3 integrin subunit resulted in a significantly (P < 0.05) lower IOP in mice within 2 weeks following the tamoxifen treatment and persisted for 11 weeks. Activating the αvβ3 integrin with the AP5 antibody resulted in a significant (P < 0.05) increase in IOP in C57BL/6J mice and a decrease in outflow facility in 42% of the POCAS.
Conclusions
These studies demonstrate a role for αvβ3 integrin signaling in the regulation of IOP.
Keywords: trabecular meshwork, glaucoma, integrins, IOP
Primary open-angle glaucoma (POAG) is a heterogeneous eye disease that is associated with irreversible damage to the optic nerve, resulting in vision loss and blindness. The most common risk factor for POAG is ocular hypertension. Approximately 30% to 40% of the population develop ocular hypertension when treated with corticosteroids for 4 to 6 weeks,1–5 and while most return to baseline when treatment stops, 1% to 3% do not, and these patients go on to develop corticosteroid-induced glaucoma.6–8 Additionally, approximately 90% of POAG patients develop ocular hypertension in response to corticosteroids.3,8–10 The etiology of both corticosteroid-induced glaucoma and POAG is believed to result from a restriction of movement of aqueous humor through the trabecular meshwork (TM) of the eye. Phenotypic changes thought to be related to the restriction in fluid movement in POAG and/or corticosteroid-induced glaucoma include the formation of cross-linked actin networks (CLANs),11 abnormal development of the extracellular matrix (ECM),12 and a decrease in phagocytosis.13
Recent studies have implicated integrins as having a role in the formation of these glaucomatous phenotypes.14 Integrins are a superfamily of glycosylated transmembrane adhesion proteins composed of an α- and a β-subunit. Their extracellular domains bind a number of ECM proteins while their cytoplasmic tails bind a variety of adaptor proteins, tyrosine kinases, and actin-binding proteins15,16 that can regulate the cytoskeletal events important for the maintenance of tissue homeostasis (ECM formation, contractility, and phagocytosis). Dysregulation of integrin signaling can disrupt this and contribute to a diseased state. One particular integrin, αvβ3 integrin, has been implicated in the pathogenesis of several diseases including cancer, diabetes, osteoporosis, and rheumatoid arthritis.17–23
Recent studies suggest that αvβ3 integrin is also the integrin responsible for the generation of several of the phenotypic changes associated with glaucoma.24–27 In particular, activation of αvβ3 integrin in TM cells causes the formation of CLANs that are believed to alter the contractile properties of TM cells.25,26,28,29 Activation of αvβ3 integrin also impairs phagocytosis in TM cells, which decreases the ability of TM cells to clear debris and degraded ECM proteins that can obstruct the aqueous humor outflow pathway across the TM.27,30 Not surprisingly, αvβ3 integrin expression and activity are implicated as playing a role in corticosteroid-induced glaucoma since it is upregulated and activated by DEX.24,27 Finally, activation of αvβ3 integrin leads to the upregulation of Hic-5, a transcription factor involved in the TGFβ2-induced fibrogenic activity of TM cells.31
While studies have shown how activation of αvβ3 integrin affects the normal activities of TM cells in culture, no studies have looked at its effect in vivo. Here we studied how expression and activation of αvβ3 integrin affect outflow facility and IOP in porcine organ culture anterior segments (POCAS) and mice. Our studies show that activation of αvβ3 integrin leads to a decrease in outflow facility in POCAS and an increase in IOP in C57BL/6J mice while a knockdown of αvβ3 integrin expression in mice causes a decrease in IOP. These are the first studies to show that an integrin known to contribute to the phenotypic changes associated with glaucoma also plays a role in regulating IOP.
Methods
Animal Studies
All animal studies were carried out in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin-Madison School of Medicine and Public Health (protocol no. M005242). The mice were housed and bred in the University of Wisconsin animal facilities with a 12-hour light/12-hour dark cycle with food and water freely available. For the antibody studies, male C57BL/6J mice were obtained from Jackson Labs (Bar Harbor, ME, USA). For the tamoxifen experiments, the original breeding pair of β3 integrinflox/flox mice (β3flox/flox) on a C57BL/6J background was kindly provided by Katherine Weilbaecher (Washington University School of Medicine, St. Louis, MO, USA). These mice were previously confirmed to be homozygous for the β3 integrinflox/flox allele, and it was shown that the β3 integrin gene (Itgb3) was fully functional.32 These mice have since been deposited with Jackson Labs (C57BL/6-Itgb3tm1.1Wlbrc/J; stock no. 028232). The tamoxifen-inducible CAGGCre-ERTM mice (B6.Cg-Tg(CAG-cre/Esr1*)5Amc/J stock no. 004682)33 were obtained from Jackson Labs. These heterozygous mice were maintained by breeding them with C57BL/6J mice (Jackson Labs). To produce Cre+/− β3flox/flox mice, mice heterozygous for CAGGCre-ERTM (Cre+/−) were crossed with homozygous β3flox/flox mice. Their littermates expressing Cre−/− β3flox/flox were used as controls. Both male and female mice were used in all experiments. Genotyping from ear punches was performed using PCR primers and thermal cycle profiles as recommended by Jackson Labs. All experiments were conducted after 6 weeks of age to allow the TM to fully develop.34
IOP Measurements
Mice (7–10 weeks of age) were anesthetized intraperitoneally with a ketamine/xylazine mix (90 mg/10 mg/kg). IOP was measured within the same 2-hour time frame (9–11 AM) 3 to 5 minutes after anesthesia administration using a rodent Icare Tonolab.35,36 Previous studies have shown that IOP is stable during this time.37–39 Three IOP measurements from each eye were averaged together at each time point.
Tamoxifen Treatment
After a baseline IOP was measured, the Cre recombinase in the Cre+/− β3flox/flox mice and their littermates was activated by treating them topically with 10 μL tamoxifen (Sigma-Aldrich Corp., St. Louis, MO, USA) diluted in corn oil (Sigma-Aldrich Corp.) to 5 mg/mL as previously described.40 The drops were given to both eyes three times a day (4 hours apart) for 5 days. Starting 2 days after the last day of tamoxifen drops, IOP was measured weekly for 11 weeks. Mice were euthanized and eyes were enucleated and processed in one of two ways. Some eyes were bisected just posterior to the limbus, and the anterior segments were lysed for Western blotting. For other eyes, a hole was poked in the sclera with a 30-gauge needle and eyes were fixed in 4% paraformaldehyde for 45 minutes at room temperature, then transferred to phosphate-buffered saline (PBS) and embedded in paraffin for immunohistochemistry (see below).
Genomic DNA Isolation and Real-Time PCR
Paraffin blocks containing tamoxifen-treated and untreated mouse anterior segments were trimmed along the parameter of the tissue using a straight-edge razor blade to minimize the amount of paraffin in the extractions. Sixteen 5-μm sections from each eye were placed in sterile, nuclease-free tubes. Genomic DNA (gDNA) was isolated using the Maxwell 16 FFPE LEV DNA Purification kit (Promega, Madison, WI, USA) and the Maxwell MDx AS3000 Instrument (Promega) following the manufacturer's instructions.
Real-time PCR using the isolated gDNA was performed using an Applied Biosystems QuantStudio 7 Flex Real-Time PCR system (Thermo Fischer Scientific, Waltham, MA, USA) with SYBR Green PCR Master Mix (Thermo Fischer Scientific). Data were normalized to succinate dehydrogenase complex flavoprotein subunit A (SDHA). Tamoxifen-treated eyes were compared to littermate control eyes for each genetic background. Primers used were β3 integrin forward 5′-AGTGGCCGGGACAACTCTG-3′ and reverse 5′-GGACTCTCCAACAACAACGC-3′ and SDHA forward 5′-GGACAACTGGAGGTGGCATT-3′ and reverse 5′-CCGTCATGTAGTGGATGGCA-3′.
Intracameral Antibody Infusion in the Mouse
After a baseline IOP was obtained, male C57BL/6J mice (9–10 weeks of age) were anesthetized as above, then given topical proparacaine HCl ophthalmic solution (0.5%; Bausch and Lomb, Rochester, NY, USA) and tropicamide ophthalmic solution (0.5%; Akorn, Lake Forest, IL, USA) to one eye to numb it and dilate the pupil, respectively. The other eye was untreated. The end of a 33-gauge needle (TSK Laboratory, Tochigi-Ken, Tochigi-Shi, Japan) with the hub removed was inserted snugly into tubing (catalog no. 427401 Intramedic PE-10 tubing; Becton Dickinson, Franklin Lakes, NJ, USA). Superglue was used to ensure the needle was secure and would not move. The needle was taped to the arm of a micrometer (catalog no. M3301L; World Precision Instruments, Sarasota, FL, USA) that was mounted on a stand (catalog no. M10; World Precision Instruments), which was magnetically attached to a metal plate on the benchtop. The tubing with the needle was attached to a 1-mL syringe placed in an infusion pump (Harvard Apparatus PHD 2000; Holliston, MA, USA) and filled with either the αvβ3 integrin-activating antibody AP541 (The Blood Center of Wisconsin, Milwaukee, WI, USA; or catalog no. EBW107, Kerafast, Inc., Boston, MA, USA) or the nonactivating αvβ3 integrin antibody AP342 (Kerafast, Inc., catalog no. EBW106) at 3.33 μg/mL. An IgG1 control antibody (ab81032; Abcam, Cambridge, MA, USA) in PBS or PBS alone was used as control. Mice were placed under a stereoscope and the needle was inserted into the cornea using the micrometer, taking care not to compromise the iris or lens. The pump was then turned on and 3 μL antibody was infused at 2.5 μL/min to deliver 10 ng antibody. The pump was then stopped and the needle remained in place for another minute before being quickly removed. Topical erythromycin ophthalmic ointment (0.5%; Bausch and Lomb) was then placed on the eye, and mice were allowed to wake up on a heating pad.
Preparation and Perfusion of Porcine Organ Culture Anterior Segment (POCAS) With AP5
Fresh porcine eyes were obtained from a local abattoir. Unpaired eyes were used in these experiments because the abattoir was unable to provide paired eyes. Anterior segments lacking the ciliary body, iris, and lens were isolated, then mounted on organ culture dishes and attached with tissue adhesive 4 to 6 hours post mortem as previously published.43,44 Briefly, one port of the culture dish was connected by tubing to a pressure transducer to monitor IOP and the other port was connected to an infusion pump. The tissue was then clamped down with an O-ring lined with silicone. The anterior segments were maintained in an incubator at 37°C in 5% CO2 and Dulbecco's modified Eagle's medium supplemented with 100 U penicillin, and 0.1 mg/mL streptomycin (Sigma-Aldrich Corp.) was infused at 4.5 μL/min using an infusion pump (Harvard Apparatus). Pressure was monitored using pressure transducers (APT300; Harvard Apparatus) attached to amplifiers (TAM-D; Harvard Apparatus) and was recorded every 5 minutes using the PowerLab (ADInstruments, Colorado Springs, CO, USA) and LabChart7 acquisition software.
POCAS were allowed to equilibrate for 48 hours to minimize the washout effect and to stabilize the IOP,43,45 during which time baseline pressure was recorded. The unpaired eyes were then randomly assigned to a treatment group. The media in one group of eyes were exchanged with 5 mL AP5 antibody at 25 μg/mL (The Blood Center of Wisconsin), which is known to activate β3 integrin.41 The other group of eyes received 25 μg/mL of an isotype-matched IgG1 control antibody (Abcam ab18447). The antibody concentration used was based on cell culture studies in our lab.46,47 For the exchange, the pumps were stopped to reload the syringe with antibody and the pressure port tubing was disconnected from the pressure transducer to allow media to enter the chamber through the in port and out to a reservoir.48 Media exchange occurred at 200 μL/min for 25 minutes, after which the tubing was reattached to the pressure transducers and perfusion with the corresponding antibody then continued at 4.5 μL/min for up to 21 hours post exchange. At the end of the experiment, eyes were removed from the organ culture plates, washed with PBS, and then fixed overnight in 4% paraformaldehyde at 4°C for post perfusion analysis (see below).
Immunohistochemistry
For the immunohistochemistry studies, the entire fixed mouse anterior segment was embedded in paraffin. In contrast, fixed POCAS segments were cut into six pieces and embedded in paraffin. The human anterior segments used in these studies were obtained from three different female donors aged 36, 46, and 93 years. None of the donors had any history of ocular disease (Wisconsin Lions Eye Bank, Madison, WI, USA). The human anterior segments were fixed and embedded in paraffin as previously described.49 For the mouse and POCAS eyes, sagittal sections 5 μm thick were cut and stained with hematoxylin 33342 and eosin to assess the morphology of the TM and whether there was any cell loss. Images were obtained using a brightfield microscope (Olympus Corp., Center Valley, PA, USA). For immunohistochemistry studies, human, mouse, and porcine sections were deparaffinized in xylenes and rehydrated through a graded series of ethanol solutions. Antigen retrieval was performed using 1 mM EDTA, pH 8.0, at 95°C for 20 minutes and sections were then blocked overnight with 1% bovine serum albumin (BSA) in PBS at 4°C as we previously described.49 The αvβ3 integrin in the human and POCAS sections was detected using the clone (BV3) mouse monoclonal antibody (Abcam ab7166) diluted 1:1000 for 2 hours at room temperature followed by Alexa 546–conjugated goat anti-mouse IgG (1:500; Thermo Fisher Scientific). Control antibodies used were anti-β-galactosidase (Sigma-Aldrich Corp. G8021) for human anterior segments and secondary antibody alone for POCAS. Sections were labeled with Hoescht 33342 to detect nuclei prior to mounting in Immu-Mount (Thermo Fisher Scientific). Levels of αvβ3 integrin in sections of mouse anterior segments were detected using a rabbit polyclonal antibody (Abcam ab197662). Rabbit IgG was used as a control. Images were acquired with an epifluorescence microscope (Zeiss International, Thornwood, NY, USA) using deconvolution and orthogonal processing software (Zeiss International).
Western Blotting
Dissected mouse anterior segments were immediately placed in 200 μL ice-cold lysis buffer (25 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM NaF, 1% NP-40, 0.25% DOC, and Halt protease and phosphatase inhibitor cocktails [Thermo Fisher Scientific]). Tissue was then sonicated (Branson Sonifier SLPe, St. Louis, MO, USA) five times for 1 second using 30% amplitude. The insoluble material was removed by centrifugation at 18,000g for 10 minutes at 4°C. A BCA assay (Thermo Fisher Scientific Pierce Micro BCA Protein Assay Kit) was used to determine protein concentration in the resulting supernatant. Proteins in the lysate (10 μg) were separated on a 4% to 20% SDS-PAGE (Bio-Rad, Hercules, CA, USA) and transferred to Immobilon-FL (MilliporeSigma, Burlington, MO, USA). The membrane was blocked with 3% BSA in Tris-buffered saline with 0.5% Tween-20 (TBST) for at least 1 hour at room temperature. Membranes were then incubated overnight at 4°C with primary antibody diluted in 1% BSA in TBST. After washing, membranes were incubated for 1 hour at room temperature with IR Dye 800–conjugated goat anti-mouse or anti-rabbit secondary antibody (Li-Cor Biosciences, Lincoln, NE, USA) diluted 1:15,000 in 1% BSA in TBST with 0.01% SDS. Membranes were washed and then digitally scanned (Odyssey CLx imager, Li-Cor). Antibodies used included mouse anti-β3 integrin clone AP3 (1:1000, catalog no. EBW106; Kerafast, Inc.), rabbit anti-β-actin (1:2000; Abcam ab8227), and rabbit anti-GAPDH (1:5000; Abcam ab9485). Densitometry of the bands was determined using Image Studio v. 5.0 software (Li-Cor).
Data Analysis
Data are presented as mean ± SEM. Statistical comparisons were done using Student's t-test, and a P value <0.05 was considered significant.
Results
Expression of αvβ3 Integrin in the Mouse and Human Anterior Segments
To determine if αvβ3 integrin plays a role in modulating IOP, we initially used a tamoxifen-inducible Cre-mouse model previously shown to be effective in altering gene expression in the anterior segment40 to create a Cre-β3 integrinflox/flox (Cre+/− β3flox/flox) mouse anterior segment deficient in αvβ3 integrin expression. The resulting Cre+/− β3flox/flox mice were healthy and fertile. They tolerated the topical tamoxifen treatments well with no weight loss and only a temporary loss of hair on the face due to grooming. The β3 integrin−/− mouse was not used because these mice have a significant (50%) embryonic lethality due to fetal hemorrhaging and placental defects, and surviving adult mice are sickly.50
As shown in Figure 1, αvβ3 integrin is expressed in the TM, sclera, and ciliary body of the mouse anterior segment. Immunohistochemistry indicated that this distribution of αvβ3 integrin in the mouse anterior segment is similar to that observed in human anterior segments. As shown in Figure 2, A through L, human eyes express αvβ3 integrin throughout the TM and within the inner wall of Schlemm's canal. It is also found in the corneal epithelium. Some sparse labeling was also observed in the sclera. Labeling of αvβ3 integrin could also be seen in the ciliary muscle and in the nonpigmented epithelium of the ciliary muscle, although this labeling was difficult to see due to the autofluorescence of the tissue as seen in the control (compare Fig. 2G versus Fig. 2H, Fig. 2J versus Fig. 2L). The similar localization pattern of αvβ3 integrin seen in the human and mouse eye indicates that mouse eyes can be used as a model to study αvβ3 integrin signaling in the conventional outflow pathway.
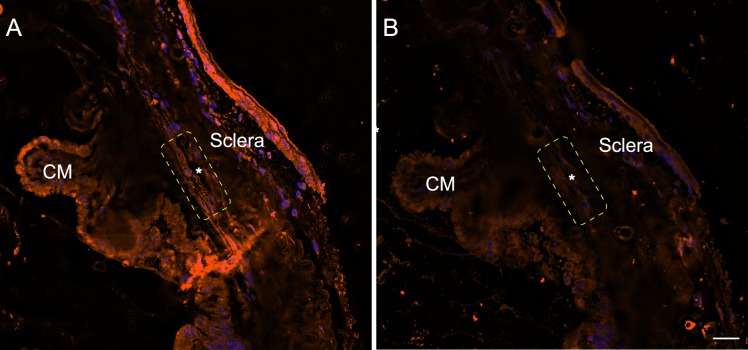
Figure 1.
Labeling of β3 integrin mouse anterior segments. Sections of mouse anterior segments labeled with rabbit antibody against β3 integrin (Abcam ab197662) (A) or a control antibody (IgG) (B). The TM-Schlemm's canal (SC) outflow pathway is outlined by the yellow dotted rectangle. The asterisk indicates SC. Micrographs are representative of several anterior segments. Micrograph shown here is from a Cre+/− β3flox/+ littermate not treated with tamoxifen. Scale bar: 20 μm.
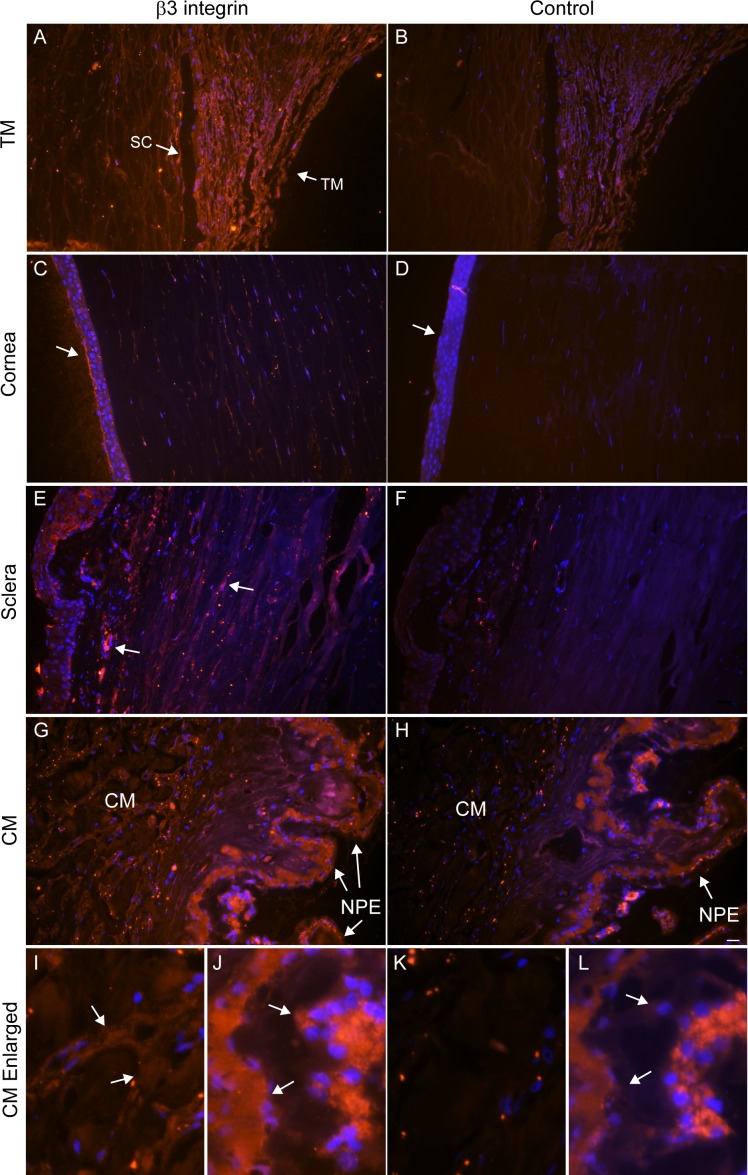
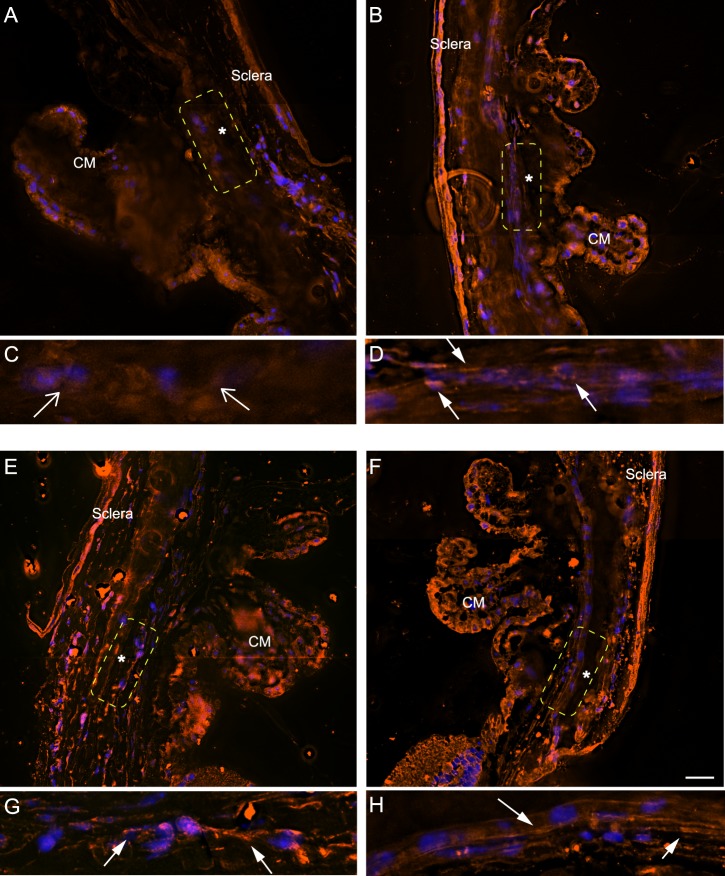
Figure 2.
Labeling of αvβ3 integrin in human anterior segments. Sections of human anterior segments from a 93-year-old donor were labeled with monoclonal antibody (BV3) against αvβ3 integrin (A, C, E, G, I, J) or a control antibody (β-galactosidase) (B, D, F, H, K, L). (A) The TM and Schlemm's canal (SC) show intense labeling for αvβ3 integrin that is clearly above the background labeling seen with the control antibody (B). (C) αvβ3 localization within the cornea. The arrow points to labeling of the corneal epithelium that is absent in the section labeled with the control antibody (D). (E) αvβ3 localization within the sclera; arrows indicate labeling absent from the section labeled with the control antibody (F). (G) αvβ3 localization within the ciliary muscle (CM) and CM nonpigmented epithelium (NPE). (H) Control antibody labeling of the same area seen in (G) that shows no labeling within the CM and NPE (arrow). (I) Enlarged view of CM shown in (G). (J) Enlarged view of NPE shown in (G). Arrows indicate positive αvβ3 labeling in these regions that is absent from the section labeled with the control antibody (K, L). Scale bar: 20 μm. Similar results were observed in anterior segments from a 36- and a 46-year-old donor (not shown).
Decreased IOP Is Observed in β3 Integrin-Deficient Mice
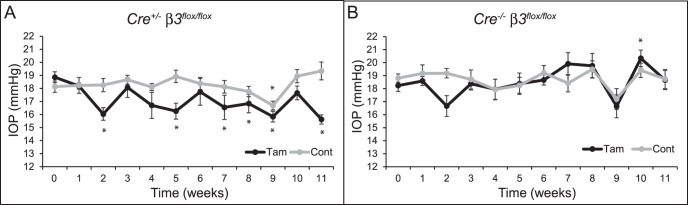
As shown in Figure 3A, when Cre+/− β3flox/flox mice were treated with tamoxifen, it resulted in a significant (P < 0.05) decrease in IOP at 2, 5, 7, 8, 9, and 11 weeks after the start of tamoxifen eye drops compared to baseline IOP. At 11 weeks after tamoxifen treatment, IOP was 3.4 ± 0.5 mm Hg below baseline IOP. In contrast, IOP in Cre+/− β3flox/flox mice not treated with tamoxifen remained fairly constant over the 11-week period and did not show any significant drop compared to baseline with the exception of the measurement at 9 weeks. By 11 weeks, the IOP in the untreated mice was 1.2 ± 0.8 mm Hg higher than baseline. IOP in Cre−/− β3flox/flox mice that do not express Cre and therefore should express normal levels of β3 integrin regardless of tamoxifen treatment was also measured (Fig. 3B). Treatment of these mice with tamoxifen had no effect on IOP, which appeared similar to that in the untreated Cre+/− β3flox/flox mice. Male and female mice responded similarly to the treatments, with no statistical differences observed between sexes (data not shown). Data from mice heterozygous for β3 integrin and/or Cre (Cre+/−β3flox/+ and Cre−/− β3flox/+) are not shown, as their IOP was similar to that in Cre+/− β3flox/flox mice not treated with tamoxifen.
Figure 3.
Knockdown of β3 integrin decreases IOP in mice. Average IOP over time of tamoxifen-treated or control (A) Cre+/− β3flox/flox mice (n = 11 tamoxifen; n = 13 control) or (B) Cre−/− β3flox/flox mice (n = 7 tamoxifen; n = 9 control). IOP is statistically significantly different from baseline IOP, *P < 0.05. Error bars are mean ± SEM.
The decrease in IOP was not due to the tamoxifen treatment affecting TM morphology. As shown in Figure 4, iridocorneal angles looked normal and open in all treatment groups. Immunolabeling studies, however, suggested that Cre+/− β3flox/flox mice treated with tamoxifen showed lower levels of αvβ3 integrin in the TM and ciliary muscle (Figs. 5A, 5C) compared to Cre+/− β3flox/flox mice not treated with tamoxifen (Figs. 5B, 5D) or Cre−/− β3flox/flox mice treated with tamoxifen (Figs. 5E, 5G) or control (Figs. 5F, 5H). Western blot analysis of the isolated anterior segments further suggested that the tamoxifen treatment altered the level of αvβ3 integrin expression in the anterior segment compared to the mice not treated with tamoxifen. As shown in Figures 6A and 6B, tamoxifen treatment caused a trend of decreased β3 integrin protein levels in the anterior segment compared to nontreated Cre+/− β3flox/flox mice. In contrast, the Cre−/− β3flox/flox mice treated with tamoxifen showed a trend in increased β3 integrin protein levels compared to no tamoxifen treatment. Neither change was, however, statistically significant compared to the no-treatment controls, which was probably due to variation in the level of β3 integrin expression observed in the entire anterior segment. Lastly, real-time PCR using gDNA isolated from paraffin-embedded anterior segments of Cre+/− β3flox/flox and Cre−/− β3flox/flox mice treated with or without tamoxifen showed a 55% decrease in the β3 integrin gene (Itgb3) in Cre+/− β3flox/flox mice treated with tamoxifen compared to the same mice not treated with tamoxifen (Fig. 6C, P < 0.05). There was no difference between Cre−/− β3flox/flox mice treated with or without tamoxifen. Together, these data suggest that the decrease in IOP could be due to a change in β3 integrin expression in the TM of Cre+/− β3flox/flox mice.
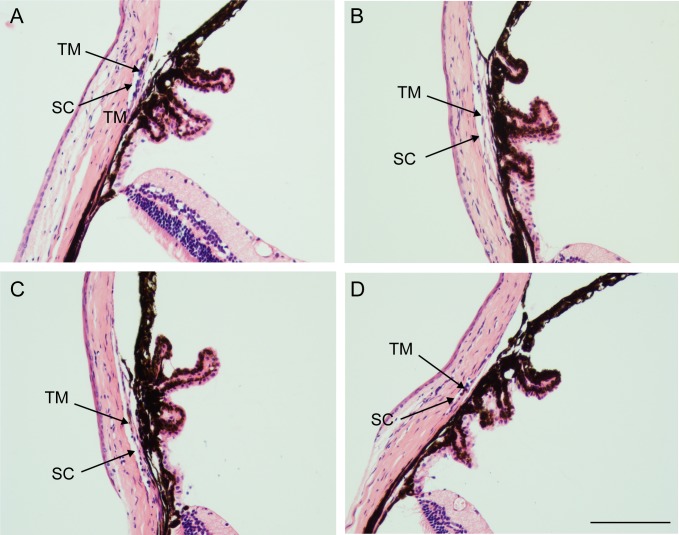
Figure 4.
Tamoxifen does not affect iridocorneal morphology. Hematoxylin and eosin staining of (A) Cre+/− β3flox/flox mouse treated with tamoxifen, (B) untreated Cre+/− β3flox/flox mouse, (C) Cre−/− β3flox/flox mouse treated with tamoxifen, and (D) untreated Cre−/− β3flox/flox mouse. Scale bar: 20 μm.
Figure 5.
β3 integrin labeling of Cre+/− β3flox/flox or Cre−/− β3flox/flox mice treated with or without tamoxifen. The TM-SC outflow pathway is outlined by the yellow dotted rectangle. The asterisk indicates SC. (A) Cre+/− β3flox/flox mouse treated with tamoxifen, (B) untreated Cre+/− β3flox/flox mouse. (C) Enlargement of the TM in (A). Open arrows indicate a lack of β3 integrin labeling. (D) Enlargement of the TM in (B). Closed arrows indicate β3 integrin labeling that is missing in (C). (E) Cre−/− β3flox/flox mouse treated with tamoxifen and (F) untreated Cre−/− β3flox/flox mouse. (G) Enlargement of the TM in (E). Closed arrows indicate β3 integrin labeling. (H) Enlargement of the TM in (F). Closed arrows indicate β3 integrin labeling. Micrographs are representative of several anterior segments. (A) n = 2, (B) n = 3, (C) n = 3, (D) n = 2. SC, Schlemm's canal. Scale bar: 20 μm.
Figure 6.
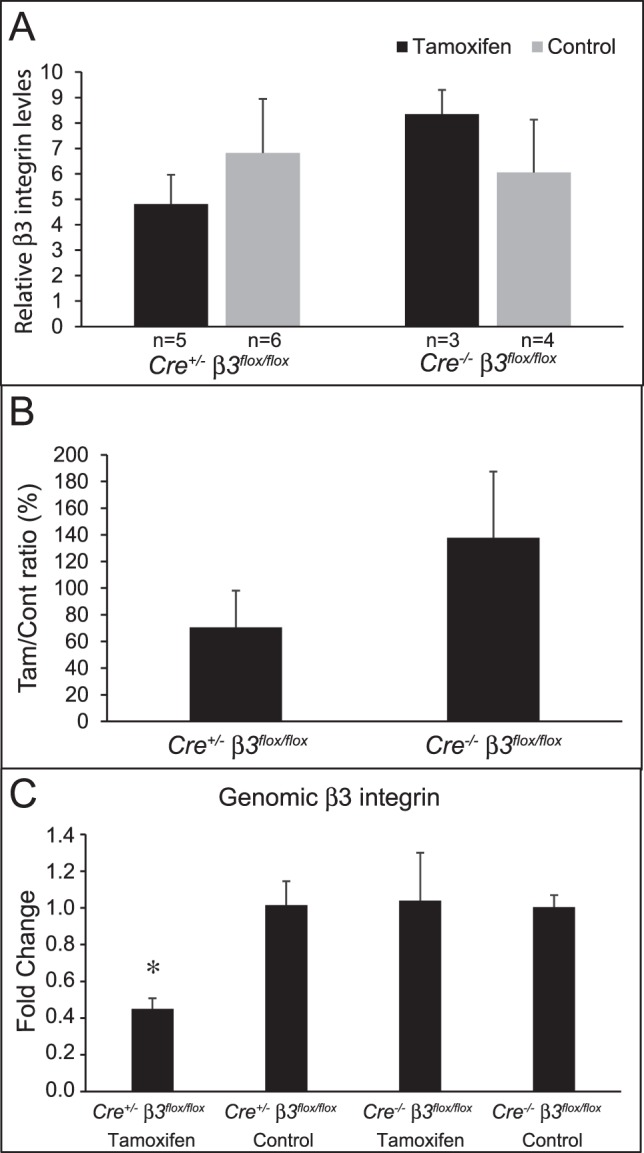
Tamoxifen treatment of Cre+/− β3flox/flox mice decreases β3 integrin expression. Anterior segments of Cre+/− β3flox/flox or Cre−/− β3flox/flox mice treated with or without tamoxifen were lysed and analyzed by Western blotting for levels of β3 integrin or the housekeeping gene GAPDH. (A) Densitometry of β3 integrin bands were normalized to GAPDH levels. (B) Ratio expressed as a percentage of tamoxifen versus control from Cre+/− β3flox/flox or Cre−/− β3flox/flox mice in (A). (C) Real-time PCR of β3 integrin using gDNA isolated from Cre+/− β3flox/flox or Cre−/− β3flox/flox mice treated with or without tamoxifen. Levels of β3 integrin in Cre+/− β3flox/flox mice treated with tamoxifen were significantly different from those in control Cre+/− β3flox/flox mice (*P < 0.05). Tam, tamoxifen, Cont, control. Error bars are mean ± SEM.
Activating αvβ3 Integrin in Mouse Eyes Increases IOP
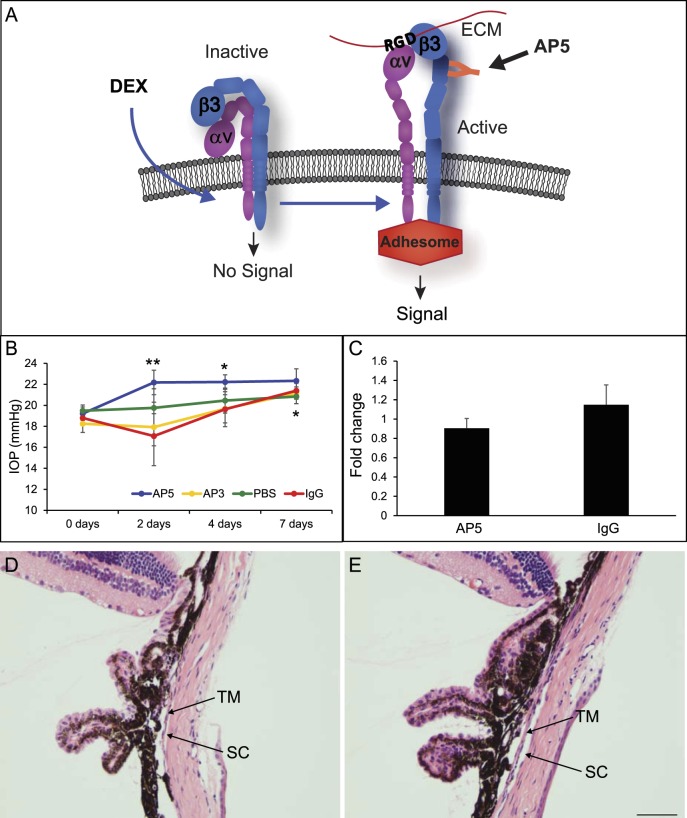
Since activation of integrins is an important step in integrin signaling, we then examined if the activation state of αvβ3 integrin had any effect on IOP. Prior studies have shown that activation of αvβ3 integrin impairs the phagocytic activity of TM cells and triggers the formation of CLANs observed in glaucomatous tissues.25–30 To trigger activation of αvβ3 integrin in vivo, we used the AP5 antibody, which has been shown to induce the active conformation of αvβ3 integrin (Fig. 7A)41 and caused the formation of CLANs in TM cells in vitro.25,26 As shown in Figure 7B, intracameral injection of AP5 into the anterior chamber of C57BL/6J mouse eyes induced a significant increase in IOP of 3.0 ± 1.2 mm Hg (P < 0.05) over baseline after 2 days, which was also significantly different from the IgG control after 2 days (AP5 22.2 ± 1.2 versus IgG 17.1 ± 0.9 mm Hg; P < 0.05). After 4 days, the IOP of AP5-treated eyes was still significantly increased (3.0 ± 0.9 mm Hg; P < 0.05) compared to baseline and remained elevated on day 7 (22.3 ± 1.1 mm Hg; P < 0.054). In contrast, mice treated with the control IgG did not show a statistically significant increase in IOP compared to baseline until day 7 (17.1 ± 0.9 vs. 21.4 ± 0.7 mm Hg, P < 0.05). By 7 days, the AP5- and IgG-treated eyes had similar elevated IOPs (22.3 ± 1.1 vs. 21.4 ± 0.7 mm Hg) compared to baseline.
Figure 7.
AP5 antibody increases IOP in C57BL/6J mice. (A) αvβ3 integrin can exist on the cell surface in both an inactive state (bent conformation) or in an active state (extended conformation). The active conformation of αvβ3 integrin can be induced by DEX24,70,71 or by the AP5 antibody, which binds to a ligand-inducible binding site in the amino terminus of the β3 subunit.41 Activation of this integrin in TM cell cultures has been shown to cause a decrease in phagocytosis and an increase in ECM and CLAN formation. (B) C57BL/6J mice were infused intracamerally with 10 ng AP5, AP3, or IgG antibody (n = 8/group or PBS alone). IOP was measured at 2, 4, and 7 days after infusion of the IgGs. IOP was significantly increased in AP5-treated eyes at day 2 compared to baseline- and IgG-treated eyes (**P < 0.05) and compared to baseline at day 4 (*P < 0.05). IOP was significantly increased in AP5-, AP3-, and IgG-treated eyes at 7 days (*P < 0.05). (C) Densitometry on Western blots of anterior segment cell lysates from treated and untreated mice showed no significant difference in αvβ3 integrin levels between AP5 and IgG treatments. Values were normalized to β-actin. (D) Hematoxylin and eosin staining of a mouse eye 7 days after IgG treatment showed that no gross changes in the anterior segment were observed. (E) Hematoxylin and eosin staining of a mouse eye 7 days after AP5 treatment also showed that no gross changes were observed.
To determine if the initial increase in IOP induced by the AP5 antibody after 2 days was specific to activation of αvβ3 integrin, we also injected the mice with another antibody called AP3 that recognizes αvβ3 integrin but does not activate it. As shown in Figure 7B, AP3 had a similar effect on IOP as the nonspecific IgG and only at 7 days post treatment was the IOP significantly different from baseline (P < 0.05). To determine if mechanical manipulation of the eye by itself could affect IOP, we injected PBS. As shown in Figure 7B, PBS has no significant effect on IOP. Finally, the increase in IOP by AP5 was not due to changes in αvβ3 integrin expression. As shown in Figure 7C, injection of AP5 or IgG had no statistically significant effect on β3 integrin levels in the anterior segment 7 days after antibody treatment, nor did it affect TM morphology. As shown in Figures 7D and 7E, the TM and Schlemm's canal looked normal in both the AP5- and IgG-treated eyes at the light microscopy level. Together these data suggest that the increase in IOP was due to the activation of αvβ3 integrin.
AP5 Increases IOP in POCAS
We then used the POCAS model to examine the effect of αvβ3 integrin activation on outflow facility. As shown in Figure 8 and the Table, treatment of POCAS for 21 hours with the αvβ3 integrin-activating antibody AP5 increased pressure in 42% of the treated POCAS (5 out of 12) while the other 58% (7 out of 12) showed no change or a decrease in pressure when compared to baseline pressure. To differentiate AP5 responders from nonresponders we calculated the 95% confidence interval of the change in IOP from baseline of IgG-treated eyes: ΔIOP ± z × SEM where z = 1.96 for 95% confidence (−1.38 ± 1.96 × 0.912 = −3.17 to 0.41 mm Hg). Based on this calculation, we considered eyes treated with AP5 that had a change in IOP from baseline greater than 0.41 mm Hg to be responders. The Table shows the POCAS data analyzed according to this criterion. In the POCAS that responded to AP5 the pressure increased by 5.9 ± 1.6 mm Hg (P < 0.05) over baseline after 21 hours of AP5 treatment, which was a 30% decrease in outflow facility when compared to baseline (0.27 ± 0.04 vs. 0.36 ± 0.04 μL/min/mm Hg; P < 0.05). In contrast, in POCAS that did not respond to AP5, pressure decreased by 2.8 ± 1.1 mm Hg compared to baseline (P > 0.05), which was a 23% increase in outflow facility when compared to baseline (0.57 ± 0.09 vs. 0.48 ± 0.08 μL/min/mm Hg; P < 0.05). A similar decrease in pressure was observed in anterior segments treated with IgG. In that case, we saw a decrease in pressure of 1.4 ± 0.91 mm Hg from baseline (P > 0.05), which was a 14% increase in outflow facility when compared to baseline (0.40 ± 0.05 vs. 0.35 ± 0.04 μL/min/mm Hg; P > 0.05). When compared to the IgG-treated control eyes, AP5-responding POCAS had significantly higher IOP (P < 0.05) and lower outflow facility (P < 0.05) while AP5 nonresponder POCAS were not significantly different from the IgG control eyes. Perfusion of the antibodies did not affect the morphology of the porcine anterior segment. As shown in Figure 9, the morphology of the anterior segment treated with AP5 and IgG showed no gross differences.
Figure 8.
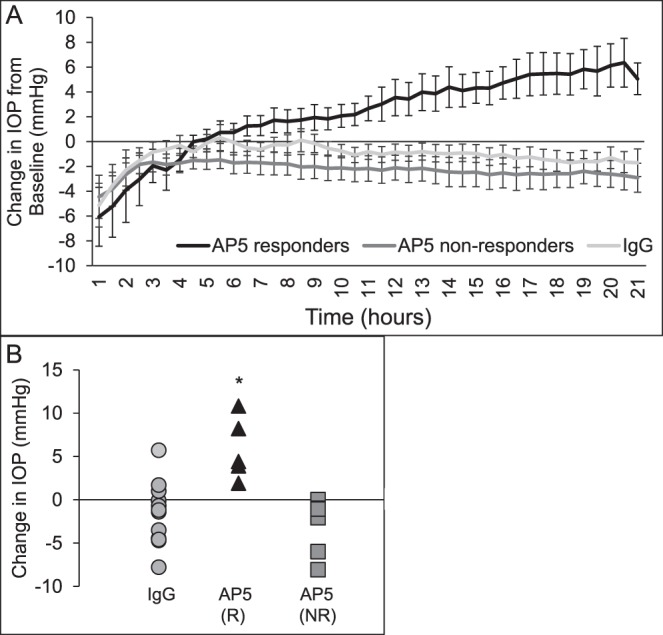
AP5 increases IOP in POCAS. After establishing a stable IOP baseline, media were exchanged with 25 μg/mL AP5 or isotype-matched IgG1 antibodies. During the exchange, the pump was stopped to reload the syringes with antibody and the tubing was opened to air to allow media to flow through the eye (Ex). Tubing was then reconnected and perfusion was continued with the antibodies for up to 21 hours. (A) Line graphs indicate the average change in IOP from baseline in POCAS that responded to AP5 with an increase in IOP (n = 5), did not respond to AP5 with an increase in IOP (n = 7), or were treated with IgG (n = 14). (B) Change in IOP from baseline after treatment with IgG or AP5 (R, responders; NR, nonresponders). IOP of AP5 (R) is significantly different from both IgG and AP5 (NR), *P < 0.05.
Table.
Individual POCAS IOP and Outflow Facility
|
Treatment |
IOP |
IOP 20 Hours |
ΔIOP |
C0 |
Cd21 |
Cd/Co |
% Change |
| AP5 responders | |||||||
| AP5 | 11.6 | 16.0 | 4.4 | 0.39 | 0.28 | 0.72 | −28 |
| AP5 | 10.0 | 13.9 | 3.9 | 0.45 | 0.32 | 0.72 | −28 |
| AP5 | 18.3 | 29.5 | 11.2 | 0.25 | 0.15 | 0.62 | −38 |
| AP5 | 13.3 | 21.4 | 8.1 | 0.34 | 0.21 | 0.61 | −39 |
| AP5 | 9.7 | 11.6 | 1.9 | 0.46 | 0.39 | 0.85 | −15 |
| Mean ± SEM | 5.9 ± 1.6* | 0.70 ± 0.04* | −30 ± 4 | ||||
| AP5 nonresponders | |||||||
| AP5 | 7.2 | 7.2 | 0 | 0.62 | 0.62 | 1.00 | 0 |
| AP5 | 9.8 | 8.9 | −0.9 | 0.46 | 0.51 | 1.11 | 11 |
| AP5 | 6.0 | 4.8 | −1.1 | 0.75 | 0.92 | 1.22 | 22 |
| AP5 | 9.1 | 7.0 | −2.1 | 0.50 | 0.64 | 1.30 | 30 |
| AP5 | 23.9 | 17.9 | −6.0 | 0.19 | 0.25 | 1.33 | 33 |
| AP5 | 7.2 | 6.1 | −1.1 | 0.63 | 0.74 | 1.17 | 17 |
| AP5 | 24.2 | 16.1 | −8.1 | 0.19 | 0.28 | 1.47 | 47 |
| Mean ± SEM | −2.8 ± 1.1 | 1.23 ± 0.06* | 23 ± 6 | ||||
| All IgG | |||||||
| IgG | 7.2 | 7.0 | −0.2 | 0.63 | 0.64 | 1.02 | 2 |
| IgG | 11.1 | 9.7 | −1.4 | 0.41 | 0.46 | 1.12 | 12 |
| IgG | 8.5 | 9.5 | 1.0 | 0.53 | 0.47 | 0.89 | −11 |
| IgG | 43.4 | 38.9 | −4.5 | 0.10 | 0.12 | 1.20 | 20 |
| IgG | 10.9 | 10.8 | −0.1 | 0.41 | 0.42 | 1.02 | 2 |
| IgG | 12.8 | 8.1 | −4.7 | 0.35 | 0.56 | 1.60 | 60 |
| IgG | 18.5 | 24.2 | 5.7 | 0.24 | 0.19 | 0.79 | −21 |
| IgG | 29.6 | 21.9 | −7.8 | 0.15 | 0.21 | 1.40 | 40 |
| IgG | 18.4 | 17.7 | −0.7 | 0.24 | 0.25 | 1.04 | 4 |
| IgG | 14.6 | 15.6 | 1.0 | 0.31 | 0.29 | 0.94 | −6 |
| IgG | 11.9 | 13.6 | 1.7 | 0.38 | 0.33 | 0.87 | −13 |
| IgG | 7.0 | 5.8 | −1.2 | 0.64 | 0.78 | 1.22 | 22 |
| IgG | 21.5 | 18.0 | −3.5 | 0.21 | 0.25 | 1.19 | 19 |
| IgG | 12.4 | 7.8 | −4.6 | 0.36 | 0.58 | 1.61 | 61 |
| Mean ± SEM | −1.4 ± 0.91 | 1.13 ± 0.07 | 14 ± 7 | ||||
IOP is measured in mm Hg. Co, average baseline outflow facility for 1 hour prior to treatment (μL/min/mm Hg); Cd21, average outflow facility from 20 to 21 hours after exchange (μL/min/mm Hg). % change is the % difference of Cd21/Co.
Significantly different from baseline at P < 0.05.
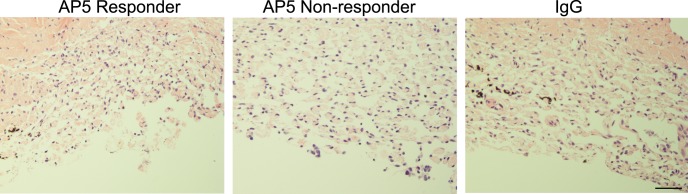
Figure 9.
AP5 does not affect POCAS TM histology. Hematoxylin and eosin staining of POCAS TM perfused with AP5 or IgG showed a similar morphology. Scale bar: 50 μm.
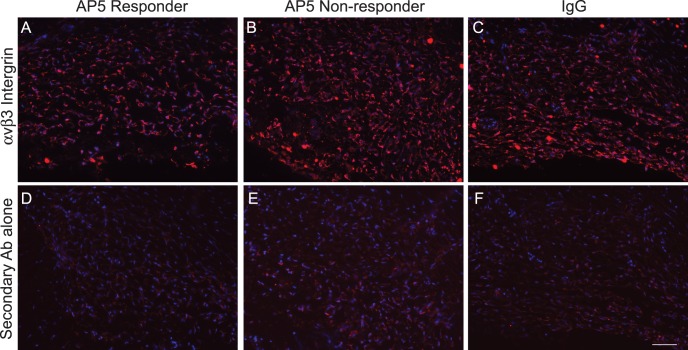
To determine if the differences between the AP5 responders and nonresponders were due to differences in αvβ3 integrin expression, we labeled paraffin-embedded POCAS with an antibody (BV3) that recognizes a different epitope from AP5 on αvβ3 integrin. As shown in Figure 10, there was strong labeling for αvβ3 integrin in the POCAS treated with both AP5 and IgG (Figs. 10A–C). Interestingly, αvβ3 integrin levels in the AP5 responders (Fig. 10A) were similar to those of the AP5 nonresponders (Fig. 10B), suggesting that the differences in response to AP5 was not due to different expression levels of αvβ3 integrin. As a control for our labeling, sections were labeled with a secondary antibody that should detect only the perfused AP5 or IgG in the TM (Figs. 10D–F); as shown, the labeling was much less than what we observed with the anti-αvβ3 integrin antibody, indicating that the αvβ3 integrin labeling seen with the (BV3) antibody was specific. Together these data suggest that activation of αvβ3 integrin can affect outflow facility, but as is the case with corticosteroid-induced glaucoma there are responders and nonresponders.1–5
Figure 10.
β3 integrin labeling of POCAS treated with AP5 or IgG. Micrographs are POCAS sections treated with AP5 (A, B, D, E) or IgG (C, F) and labeled with monoclonal antibody (BV3) that detects endogenous αvβ3 integrin (A–C) or a secondary antibody to detect the perfused AP5 or IgG (D–F). αvβ3 integrin labeling is similar in all treatment groups. Scale bar: 50 μm.
Discussion
Enhanced expression and activation of the αvβ3 integrin by dexamethasone (DEX) in human TM cells in culture have been shown to generate the phenotypic changes commonly associated with corticosteroid-induced glaucoma and shared with POAG.24–27 In this study we extended those findings and show that altering both the expression levels and activity of αvβ3 integrin signaling can influence IOP in vivo and in cultured anterior segments in situ. Using a tamoxifen-inducible Cre+/− β3flox/flox mouse model to reduce the expression of αvβ3 integrin in the anterior segment, we showed that lowering the level of αvβ3 integrin expression resulted in a reduction in IOP. In contrast, using an αvβ3 integrin-activating antibody that promotes the active conformation of αvβ3 integrin, we were able to cause an increase in IOP in all C57BL/6J mice in vivo and in 42% of the POCAS studied. To our knowledge this is the first time that an integrin has been shown to play a role in modulating IOP.
Surprisingly, only a small decrease in αvβ3 integrin expression levels was detected in Western blot analysis of lysed anterior segments, yet we were able to observe a statistically significant decrease in IOP. The failure to detect a statistically significant decrease in αvβ3 integrin expression may be due to the relatively low levels of αvβ3 integrin expressed in the TM compared to the ciliary body. Thus, decreases in the expression of αvβ3 integrin in the TM may not be detectable by Western blot analysis of the entire anterior segment given that its expression is so low compared to that in the ciliary body. Nevertheless, we were able to see a decrease in αvβ3 integrin protein expression in the TM by immunofluorescence microscopy and at the genomic level by real-time PCR, which suggests that decreasing αvβ3 integrin expression even by a small amount could cause a statistically significant decrease in IOP. The idea that small changes in integrin expression could affect TM function is not unexpected. Previous microarray analysis of DEX-treated HTM cells showed only small differences (<2-fold changes) in αvβ3 integrin gene expression.51,52 Yet this change in αvβ3 integrin expression in these cells clearly triggered the glaucomatous phenotype of TM cells.24–27
The increase in IOP caused by AP5 at days 2 and 4 was most likely due to specific interactions between AP5 and the αvβ3 integrin and not a nonspecific interaction with Fc receptors on the cell surface. If a nonspecific interaction with Fc receptors was involved we would have seen a similar increase in IOP with the control IgG at days 2 and 4, since the control and AP5 antibodies were the same isotype and likely would interact with the same Fc receptors.53,54 In addition, another αvβ3 integrin antibody (AP3) that does not activate integrin did not increase IOP until 7 days after treatment, similar to the IgG control, suggesting there may be a general antibody effect on IOP that does not manifest until 7 days after the initial injection. The increase in IOP caused by AP5 could not have been due to the AP5 “clogging” the outflow pathway, since the same amount of antibody (10 ng) was infused into all the eyes. In addition, while it is unknown for mouse aqueous humor, human aqueous humor contains 7 mg/100 mL IgG,55 which is far more than was administered to mice in this study. Thus, the most likely explanation is that the AP5 activated the αvβ3 integrin. It is unknown why both the nonactivating αvβ3 integrin antibody AP3 and the control IgG increased IOP over time in the C57BL6/J mice. The AP5, AP3, and IgG antibodies were derived in mice and should not have induced a significant immunologic response in mice. The delayed increase in IOP compared to that observed with AP5, however, suggests that the mechanism by which AP3 and IgG increased IOP in mice was a different mechanism than the one induced in mice treated with AP5 and may be a generic antibody response.
It is interesting that some of the POCAS failed to respond to the activating AP5 antibody because this is similar to what is observed in corticosteroid-induced glaucoma in humans and indicates that in vivo there may be porcine responders and nonresponders.1–5 The cause for the nonresponse in some of the POCAS is not known and could be due to differences in the porcine breed, age, and sex, since the eyes were obtained over several months from an abattoir that dealt with different breeds of pigs for which we were unable to get any information. This type of variability would be consistent with previous reports using mice on a mixed background, humans, nonhuman primates, and bovines to study corticosteroid-induced glaucoma.56–59 In those studies, only 30% to 40% of the animals responded to corticosteroids with an elevation in IOP.60 This was in contrast to the mice injected intracamerally with AP5 with all the mice responding with an increase in IOP. The different result obtained with our mice compared to POCAS is mostly like due to the inbred nature of the mice we used.
The failure to elicit a response in 58% of the POCAS is most likely due to the inherent nature of integrin signaling and not the expression of αvβ3 integrin as we showed. It is well established that integrin activity is a very complex process because it is highly dependent on its conformation, microenvironment, and various binding partners61–63 or cross-talk with other ligand-engaged integrins on the cell surface.64 For instance, there are over 60 proteins that have been found in the integrin adhesion complex when the integrin is engaged by fibronectin, and changes in any one of them can influence the downstream targets of integrins.65,66 Hence differences between the ECM composition, repertoire of integrins on the cell surface, and cytoplasmic components of nonresponders and responders could be affecting αvβ3 integrin signaling.
In conclusion, it is not entirely surprising that αvβ3 integrin would play a role in regulating IOP. Its signaling pathways have been shown to trigger the changes frequently cited to cause a restriction in outflow facility. In addition, changes in its expression and/or activity, which are normally lower in adult tissue and vary in the adult TM,14 have also been shown to play a role in the pathogenesis of a number of human diseases in adults.67 Together these data suggest that individuals with higher levels of αvβ3 integrins and/or more activated αvβ3 integrins would be more likely to respond to steroids and/or develop glaucoma. Understanding the role integrins play in regulating IOP could open up new therapeutic treatments for glaucoma utilizing some of the current anti-integrin therapies used to treat other ocular diseases.68,69
Acknowledgments
The authors thank the University of Wisconsin Translational Research Initiatives in Pathology laboratory (TRIP), supported by the UW Department of Pathology and Laboratory Medicine, UWCCC (P30 CA014520), and the Office of The Director–National Institutes of Health (S10OD023526) for their assistance with the gDNA analysis and histology.
Supported by National Eye Institutes Grants EY017006, EY026009 (DMP), and a Core Grant to the Department of Ophthalmology and Visual Sciences (P30 EY016665).
Disclosure: J.A. Faralli, None; M.S. Filla, None; D.M. Peters, None
References
- 1.Armaly MF. Effect of corticosteroids on intraocular and fluid dynamics. I. The effect of dexamethasone in the normal eye. Arch Ophthalmol. 1963;70:482–491. doi: 10.1001/archopht.1963.00960050484010. [DOI] [PubMed] [Google Scholar]
- 2.Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. II. The effect of dexamethasone in the glaucomatous eye. Arch Ophthalmol. 1963;70:492–499. doi: 10.1001/archopht.1963.00960050494011. [DOI] [PubMed] [Google Scholar]
- 3.Becker B, Mills DW. Corticosteroids and intraocular pressure. Arch Ophthalmol. 1963;70:500–507. doi: 10.1001/archopht.1963.00960050502012. [DOI] [PubMed] [Google Scholar]
- 4.Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752–759. doi: 10.1016/j.exer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Dibas A, Yorio T. Glucocorticoid therapy and ocular hypertension. Eur J Pharmacol. 2016;787:57–71. doi: 10.1016/j.ejphar.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espildora J, Vicuna P, Diaz E. Cortisone-induced glaucoma: a report on 44 affected eyes. J Fr Ophthalmol. 1981;4:503–508. [PubMed] [Google Scholar]
- 7.Francois J. Corticosteroid glaucoma. Ann Ophthalmol. 1977;9:1075–1080. [PubMed] [Google Scholar]
- 8.Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye. 2006;20:407–416. doi: 10.1038/sj.eye.6701895. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein HN, Mills DW, Becker B. Steroid-induced elevation of intraocular pressure. Arch Ophthalmol. 1963;70:15–18. doi: 10.1001/archopht.1963.00960050017005. [DOI] [PubMed] [Google Scholar]
- 10.Jones R, III,, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163–167. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- 11.Clark AF, Miggans ST, Wilson K, Browder S, McCartney MD. Cytoskeletal changes in cultured human glaucoma trabecular meshwork cells. J Glaucoma. 1995;4:183–188. [PubMed] [Google Scholar]
- 12.Tektas O-Y, Lutjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88:769–775. doi: 10.1016/j.exer.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto Y, Johnson DH. Dexamethasone decreases phagocytosis by human trabecular meshwork cells in situ. Invest Ophthalmol Vis Sci. 1997;38:1902–1907. [PubMed] [Google Scholar]
- 14.Filla MS, Faralli JA, Peotter JL, Peters DM. The role of integrins in glaucoma. Exp Eye Res. 2017;158:124–136. doi: 10.1016/j.exer.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brakebusch C, Fassler R. The integrin-actin connection, an eternal love affair. EMBO J. 2003;22:2324–2333. doi: 10.1093/emboj/cdg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legate KR, Fassler R. Mechanisms that regulate adaptor binding to β-integrin cytoplasmic tails. J Cell Sci. 2009;122:187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- 17.Alghisi GC, Ruegg C. Vascular integrins in tumor angiogenesis: mediators and therapeutic targets. Endothelium. 2006;13:113–135. doi: 10.1080/10623320600698037. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Park R, Tohme M, Shahinian AH, Bading JR, Conti PS. MicroPET and autoradiographic imaging of breast cancer alpha v-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconjug Chem. 2004;15:41–49. doi: 10.1021/bc0300403. [DOI] [PubMed] [Google Scholar]
- 19.Clemmons DR, Maile LA, Ling Y, Yarber J, Busby WH. Role of the integrin αvβ3 in mediating increased smooth muscle cell responsiveness to IGF-1 in response to hyperglycemic stress. Growth Horm IGF Res. 2007;17:265–270. doi: 10.1016/j.ghir.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchinson JH, Halczenko W, Brashear KM, et al. Nonpeptide αvβ3 antagonists. 8. In vitro and in vivo evaluation of a potent αvβ3 antagonist for the prevention and treatment of osteoporosis. J Med Chem. 2003;46:4790–4798. doi: 10.1021/jm030306r. [DOI] [PubMed] [Google Scholar]
- 21.Kuphal S, Bauer R, Bosserhoff AK. Integrin signaling in malignant melanoma. Cancer Metastasis Rev. 2005;24:195–222. doi: 10.1007/s10555-005-1572-1. [DOI] [PubMed] [Google Scholar]
- 22.Panchatcharam M, Miriyala S, Yang F, et al. Enhanced proliferation and migration of vascular smooth muscle cells in response to vascular injury under hyperglycemic conditions is controlled by β3 integrin signaling. Int J Biochem Cell Biol. 2010;42:965–974. doi: 10.1016/j.biocel.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilder RL. Integrin alpha v beta 3 as a target for treatment of rheumatoid arthritis and related rheumatic diseases. Ann Rheum Dis. 2002;61(suppl 2):96–99. doi: 10.1136/ard.61.suppl_2.ii96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faralli JA, Gagen D, Filla MS, Crotti TN, Peters DM. Dexamethasone increases αvβ3 integrin expression and affinity through a calcineurin/NFAT pathway. Biochim Biophys Acta. 2013;1833:3306–3313. doi: 10.1016/j.bbamcr.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filla MS, Schwinn MK, Nosie AK, Clark RW, Peters DM. Dexamethasone-associated cross-linked actin network formation in human trabecular meshwork cells involves β3 integrin signaling. Invest Ophthalmol Vis Sci. 2011;52:2952–2959. doi: 10.1167/iovs.10-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filla MS, Schwinn MK, Sheibani N, Kaufman PL, Peters DM. Regulation of cross-linked actin network (CLAN) formation in human trabecular meshwork (HTM) cells by convergence of distinct β1 and β3 integrin pathways. Invest Ophthalmol Vis Sci. 2009;50:5723–5731. doi: 10.1167/iovs.08-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gagen D, Filla MS, Clark RW, Liton P, Peters DM. Activated αvβ3 integrin regulates αvβ5 integrin-mediated phagocytosis in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2013;54:5000–5011. doi: 10.1167/iovs.13-12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark AF, Brotchie D, Read AT, et al. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskeleton. 2005;60:83–95. doi: 10.1002/cm.20049. [DOI] [PubMed] [Google Scholar]
- 29.Filla MS, Woods A, Kaufman PL, Peters DM. β1 and β3 integrins cooperate to induce syndecan-4-containing cross-linked actin networks in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47:1956–1967. doi: 10.1167/iovs.05-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peotter JL, Phillips J, Tong T, Dimeo K, Gonzalez JM, Jr,, Peters DM. Involvement of Tiam1, RhoG and ELMO2/ILK in Rac1-mediated phagocytosis in human trabecular meshwork cells. Exp Cell Res. 2016;347:301–311. doi: 10.1016/j.yexcr.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pattabiraman PP, Rao PV. Hic-5 regulates actin cytoskeletal reorganization and expression of fibrogenic markers and myocilin in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2015;56:5656–5669. doi: 10.1167/iovs.15-17204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan EA, Schneider JG, Baroni TE, et al. Dissection of platelet and myeloid cell defects by conditional targeting of the beta3-integrin subunit. FASEB J. 2010;24:1117–1127. doi: 10.1096/fj.09-138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 34.Smith RS, Zabaleta A, Savinova OV, John SW. The mouse anterior chamber angle and trabecular meshwork develop without cell death. BMC Dev Biol. 2001;1:3. doi: 10.1186/1471-213X-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pease ME, Cone FE, Gelman S, Son JL, Quigley HA. Calibration of the TonoLab tonometer in mice with spontaneous or experimental glaucoma. Invest Ophthalmol Vis Sci. 2011;52:858–864. doi: 10.1167/iovs.10-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson TV, Fan S, Toris CB. Rebound tonometry in conscious, conditioned mice avoids the acute and profound effects of anesthesia on intraocular pressure. J Ocul Pharmacol Ther. 2008;24:175–185. doi: 10.1089/jop.2007.0114. [DOI] [PubMed] [Google Scholar]
- 37.Cahatterjee A, Oh D-J, Kang MH, Rhee DJ. Central corneal thickness does not correlate with TonoLab-measured IOP in several mouse strains with single transgenic mutations of matricellular proteins. Exp Eye Res. 2013;115:106–112. doi: 10.1016/j.exer.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savinova OV, Sugiyama F, Martin JE, et al. Intraocular pressure in genetically distinct mice: an update and strain survey. BMC Genet. 2001;2:12. doi: 10.1186/1471-2156-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aihara M, Lindsey JD, Weinreb RN. Twenty-four hour pattern of mouse intraocular pressure. Exp Eye Res. 2003;77:681–686. doi: 10.1016/j.exer.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Schlecht A, Leimbeck SV, Tamm ER, Braunger BM. Tamoxifen-containing eye drops successfully trigger Cre-mediated recombination in the entire eye. Adv Exp Med Biol. 2016;854:495–500. doi: 10.1007/978-3-319-17121-0_66. [DOI] [PubMed] [Google Scholar]
- 41.Honda S, Tomiyama Y, Pelletier AJ, et al. Topography of ligand-induced binding sites, including a novel cation-sensitive epitope (AP5) at the amino terminus, of the human integrin beta 3 subunit. J Biol Chem. 1995;270:11947–11954. doi: 10.1074/jbc.270.20.11947. [DOI] [PubMed] [Google Scholar]
- 42.Van Agthoven JF, Xiong J-P, Alonso JL, et al. Structural basis for pure antagonism of integrin αvβ3 by a high affinity form of fibronectin. Nat Struct Mol Biol. 2014;21:383–388. doi: 10.1038/nsmb.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bachmann B, Birke M, Kook D, Eichhorn M, Lutjen-Drecoll E. Ultrastructural and biochemical evaluation of the porcine anterior chamber perfusion model. Invest Ophthalmol Vis Sci. 2006;47:2011–2020. doi: 10.1167/iovs.05-1393. [DOI] [PubMed] [Google Scholar]
- 44.Johnson DH, Tschumper RC. Human trabecular meshwork organ culture. A new method. Invest Ophthalmol Vis Sci. 1987;28:945–953. [PubMed] [Google Scholar]
- 45.Waxman S, Wang C, Dang Y, et al. Structure-function changes of the porcine distal outflow tract in response to nitric oxide. Invest Ophthalmol Vis Sci. 2018;59:4886–4895. doi: 10.1167/iovs.18-24943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faralli JA, Newman JR, Sheibani N, Dedhar S, Peters DM. Integrin-linked kinase regulates integrin signaling in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:1684–1692. doi: 10.1167/iovs.10-6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwinn MK, Gonzalez JM, Jr,, Gabelt BT, Sheibani N, Kaufman PL, Peters DM. Heparin II domain of fibronectin mediates contractility through an α4β1 co-signaling pathway. Exp Cell Res. 2010;316:1500–1512. doi: 10.1016/j.yexcr.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhattacharya SK, Gabelt BT, Ruiz J, Picciani R, Kaufman PL. Cochlin expression in anterior segment organ culture models after TGFβ2 treatment. Invest Ophthalmol Vis Sci. 2009;50:551–559. doi: 10.1167/iovs.08-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Filla MS, David G, Weinreb RN, Kaufman PL, Peters DM. Distribution of syndecans 1-4 within the anterior segment of the human eye: expression of a variant syndecan-3 and matrix-associated syndecan-2. Exp Eye Res. 2004;79:61–74. doi: 10.1016/j.exer.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, et al. β3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark R, Nosie AK, Walker T, et al. Comparative genomic and proteomic analysis of cytoskeletal changes in dexamethasone-treated trabecular meshwork cells. Mol Cell Proteomics. 2013;12:194–206. doi: 10.1074/mcp.M112.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rozsa FW, Reed DM, Scott KM, et al. Gene expression profile of human trabecular meshwork cells in response to long-term dexamethasone exposure. Mol Vis. 2006;12:125–141. [PubMed] [Google Scholar]
- 53.Swisher JF, Feldman GM. The many faces of FcgRI: implications for therapeutic antibody function. Immunol Rev. 2015;268:160–174. doi: 10.1111/imr.12334. [DOI] [PubMed] [Google Scholar]
- 54.Nimmerjahn F, Ravetch JV. Fcg receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 55.Sen DK, Sarin GS, Saha K. Immunoglobulins in human aqueous humour. Br J Ophthalmol. 1977;61:216–217. doi: 10.1136/bjo.61.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clark AF, Wilson K, de Kater AW, Allingham RR, McCartney MD. Dexamethasone-induced ocular hypertension in perfusion-cultured human eyes. Invest Ophthalmol Vis Sci. 1995;36:478–489. [PubMed] [Google Scholar]
- 57.Mao W, Tovar-Vidales T, Yorio T, Wordinger RJ, Clark AF. Perfusion-cultured bovine anterior segments as an ex vivo model for studying glucocorticoid-induced ocular hypertension and glaucoma. Invest Ophthalmol Vis Sci. 2011;52:8068–8075. doi: 10.1167/iovs.11-8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fingert JH, Clark AF, Craig JE, et al. Evaluation of the myocilin (MYOC) glaucoma gene in monkey and human steroid-induced ocular hypertension. Invest Ophthalmol Vis Sci. 2001;42:145–152. [PubMed] [Google Scholar]
- 59.Whitlock NA, McKnight B, Corcoran KN, Rodriguez LA, Rice DS. Increased intraocular pressure in mice treated with dexamethasone. Invest Ophthalmol Vis Sci. 2010;51:6496–6503. doi: 10.1167/iovs.10-5430. [DOI] [PubMed] [Google Scholar]
- 60.Rybkin I, Gerometta R, Fridman G, Candia O, Danias J. Model systems for the study of steroid-induced IOP elevation. Exp Eye Res. 2017;158:51–58. doi: 10.1016/j.exer.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim C, Ye F, Ginsberg MH. Regulation of integrin activation. Annu Rev Cell Dev Biol. 2011;27:321–345. doi: 10.1146/annurev-cellbio-100109-104104. [DOI] [PubMed] [Google Scholar]
- 62.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson SD, Hodivala-Dilke KM. The role of β3-integrins in tumor angiogenesis: context is everything. Curr Opin Cell Biol. 2011;23:630–637. doi: 10.1016/j.ceb.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez AM, Bhattacharya R, deHart GW, Jones JC. Transdominant regulation of integrin function: mechanisms of crosstalk. Cell Signal. 2010;22:578–583. doi: 10.1016/j.cellsig.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Humphries JD, Chastney MR, Askari JA, Humphries MJ. Signal transduction via integrin adhesion complexes. Curr Opin Cell Biol. 2019;56:14–21. doi: 10.1016/j.ceb.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Horton ER, Byron A, Askari JA, et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat Cell Biol. 2015;17:1577–1587. doi: 10.1038/ncb3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weis SM, Cheresh DA. αv integrins in angiogenesis and cancer. Cold Spring Harb Perspect Med. 2011;1:a006478. doi: 10.1101/cshperspect.a006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalez-Salinas R, Hernandez-Zimbron LF, Guilas-Canizo R, et al. Current anti-integrin therapy for ocular disease. Semin Ophthalmol. 2018;33:634–642. doi: 10.1080/08820538.2017.1388411. [DOI] [PubMed] [Google Scholar]
- 69.Tolentino MJ. Current molecular understanding and future treatment strategies for pathologic ocular neovascularization. Curr Mol Med. 2009;9:973–981. doi: 10.2174/156652409789712783. [DOI] [PubMed] [Google Scholar]
- 70.Filla MS, Dimeo KD, Tong T, Peters DM. Disruption of fibronectin matrix affects type IV collagen, fibrillin and laminin deposition into extracellular matrix of human trabecular meshwork (HTM) cells. Exp Eye Res. 2017;165:7–19. doi: 10.1016/j.exer.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Faralli JA, Clark RW, Filla MS, Peters DM. NFATc1 activity regulates the expression of myocilin induced by dexamethasone. Exp Eye Res. 2015;130:9–16. doi: 10.1016/j.exer.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]