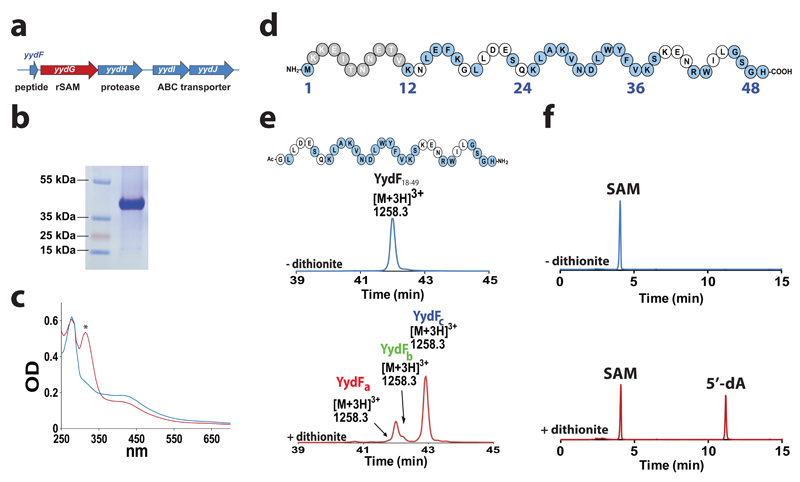
Figure 1. YydG is a radical SAM enzyme catalyzing the modification of the YydF peptide.
(a) Structure of the yydFGHIJ operon. yydF: putative peptide, yydG: radical SAM enzyme, yydH: protease, yydIJ ABC-type transporter. (b) Gel electrophoresis analysis of purified YydG expressed in E. coli. (c) UV-visible spectrum of anaerobically reconstituted YydG (blue line) and reduced with sodium dithionite (red line). The symbol * indicates absorbance due to reduced sodium dithionite. (d) Sequence of the YydF peptide from B. subtilis. In grey, region with a low conservation; in blue, strictly conserved amino acid residues (see Supplementary Fig. S2). (e) HPLC analysis of YydF18-49 incubated with YydG after 90 min under anaerobic conditions in the absence (upper trace) or the presence (lower trace) of sodium dithionite as one-electron donor. In the absence of sodium dithionite (upper trace), only the YydF18-49 peptide substrate was monitored. The m/z of each peptide is indicated above the corresponding peaks. See Supplementary Information for experimental conditions. (f) HPLC analysis of SAM incubated with YydG and YydF18-49 after 90 min under anaerobic conditions in the absence (upper trace) or in the presence (lower trace) of sodium dithionite. See Supplementary Information for experimental conditions.