Figure 2.
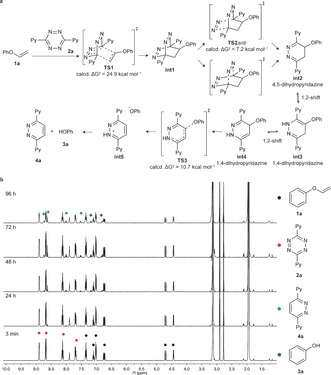
a) Proposed mechanism based on quantum mechanics for the IEDDA cycloaddition of 1 a and 2 a, followed by in situ alcohol release. Only the relevant activation free energies (ΔG ≠) are shown. The initial cycloaddition is the rate‐limiting step. After very fast nitrogen cleavage, different dihidropyridazine tautomers int2–int4 equilibrate before irreversibly decaging to the experimentally obtained products (3 a and 4 a). See Figure S4 in the Supporting Information for the whole calculated minimum energy pathway. b) 1H NMR release studies of 1 a upon reaction with 2 a. The reaction was performed at 3 mm of 1 a and 2 a in 10 % D2O/CD3CN. The reaction was monitored for 96 h. While the reaction was not always complete at 96 h, the results obtained were consistent with the mechanism supported by the theoretical calculations. Ph=phenyl, Py=pyridine.
