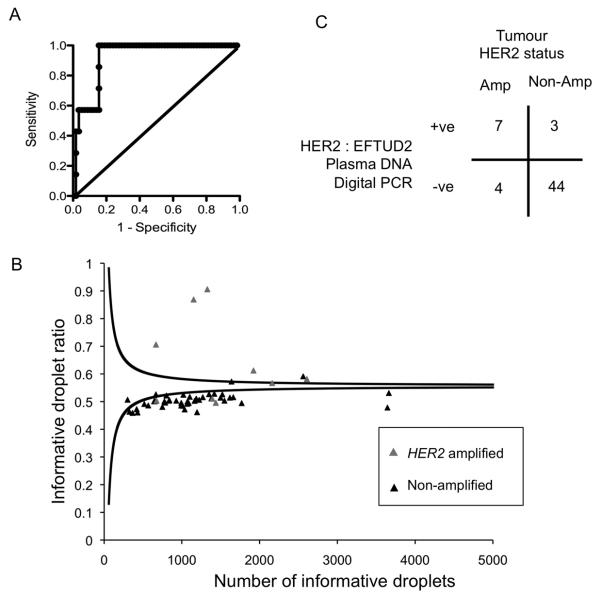
Figure 4. Plasma DNA digital PCR with the EFTUD2 reference probe has high diagnostic accuracy in an independent cohort.
A. Development cohort for EFTUD2 reference probe. Plasma DNA digital PCR HER2:EFTUD2 ratio was assessed in plasma samples from 65 patients with metastatic breast cancer, consisting of 7 with HER2 amplified cancer and 58 with HER2 non-amplified cancer. ROC analysis with an AUC 0.92 (95% CI 0.86 to 0.99, P=0.0003). A cut-off of 1.25 was selected to define HER2 amplification in the validation series.
B. Assessment of HER2:EFTUD2 in an independent validation cohort. Plasma DNA digital PCR results analysed by SPRT on plasma samples from 11 patients with HER2 amplified cancers and 47 patients with HER2 non-amplified cancers. Grey triangle indicates patients with HER2 amplified tumours and black triangle HER2 non-amplified tumours. The displayed SPRT decision boundaries are for illustrative purposes only, as the exact level varies according to the EFTUD2 control probe concentration (MEFTUD2), with the displayed boundaries calculated with MEFTUD2=0.025. Cases with a number of informative droplets > 5000 are not displayed.
C. Tabulated results of Plasma DNA digital PCR analysed by SPRT on independent validation cohort. p=0.0001 Fishers Exact test.