Fig. 5. The apparent reaction order is controlled by the surface saturation.
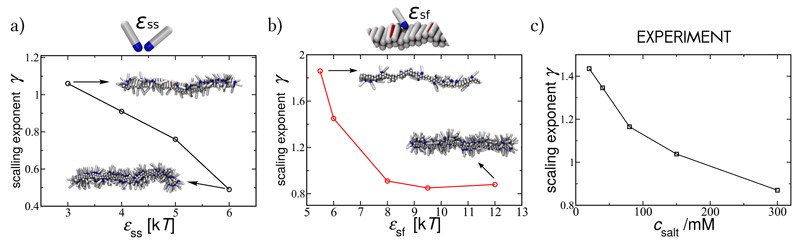
Simulation results: (a) Scaling exponent for the kinetics of fibril self-replication, averaged over the range of concentrations (20μM ≤ c ≤ 1mM), as a function of the interpeptide interaction between soluble monomers at constant peptide-fibril affinity ϵsf = 8kT, and (b) as a function of the peptide-fibril affinity at constant inter-peptide affinity ϵss = 4kT. An increase in ϵss and ϵsf increases the surface coverage, as shown by the representative snapshots in insets, taken at a monomer concentration c = 0.15mM. Experimental results: (c) The average scaling exponent for self-replication of Aβ42 fibrils at a range of NaCl concentrations, whose increase is expected to increase both ϵss and ϵsf from Ref. [18].