Abstract
Background
Symptomatic vitreomacular adhesion (sVMA) is a recognised cause of visual loss and by tradition has been managed by pars plana vitrectomy (PPV). A less invasive alternative to surgery in some people is enzymatic vitreolysis, using an intravitreal injection of ocriplasmin.
Objectives
To assess the efficacy and safety of ocriplasmin compared to no treatment, sham or placebo for the treatment of sVMA.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2017, Issue 1), MEDLINE Ovid (1946 to 24 February 2017), Embase Ovid (1947 to 24 February 2017), PubMed (1946 to 24 February 2017), the ISRCTN registry (www.isrctn.com/editAdvancedSearch); searched 24 February 2017, ClinicalTrials.gov (www.clinicaltrials.gov); searched 24 February 2017 and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en); searched 24 February 2017. We did not use any date or language restrictions in the electronic searches for trials.
Selection criteria
We included randomised controlled trials (RCTs) of people with sVMA. The intervention was intravitreal ocriplasmin 125 μg injection, and this was compared to placebo or sham injection (control). Placebo was defined as a single intravitreal injection of 0.10 mL placebo with identical drug vehicle diluted with saline. A sham injection was defined as the syringe hub or blunt needle touching the conjunctiva to simulate an injection.
Data collection and analysis
Two authors independently selected relevant trials, assessed methodological quality and extracted data. We graded the certainty of the evidence using the GRADE approach.
Main results
This review included four RCTs conducted in Europe and the USA with a total of 932 eyes of 932 participants. Participants were 18 to 97 years of age, with evidence of focal vitreomacular adhesion (VMA) on optical coherence tomography (OCT) imaging, with a best corrected visual acuity (BCVA) of 20/25 or worse in the study eye and 20/400 or better in the fellow eye. The interventions compared were intravitreal ocriplasmin versus sham (two RCTs) or placebo (two RCTs) injection. Both sham and placebo injection were classified as the control group. The main outcome measures were assessed at 28 days and six months. Overall, we judged the studies to have a low or unclear risk of bias. All four RCTs were sponsored by the manufacturers of ocriplasmin.
Compared with control, ocriplasmin treatment was more likely to result in VMA release within 28 days (risk ratio (RR) 3.46, 95% confidence interval (CI) 2.00 to 6.00; 859 eyes, 4 RCTs, high‐certainty evidence). Approximately 97/1000 eyes will have VMA release within 28 days without treatment. An additional 237 eyes will have VMA release within 28 days for every 1000 eyes treated with ocriplasmin (95% CI 96 more to 482 more).
Treatment with ocriplasmin was also more likely to result in macular hole closure (RR 2.87, 95% CI 1.50 to 5.51; 229 eyes, 3 RCTs, high‐certainty evidence). Approximately 123/1000 eyes with macular holes will have closure with no treatment. An additional 231 eyes will have macular hole closure for every 1000 eyes treated with ocriplasmin (95% CI 62 more to 556 more).
Eyes receiving ocriplasmin were also more likely to have complete posterior vitreous detachment (PVD) within 28 days (RR 2.94, 95% CI 1.39 to 6.24; 689 eyes, 3 RCTs, high‐certainty evidence). Approximately 40/1000 eyes will have complete PVD within 28 days without treatment. An additional 78 eyes will have complete PVD within 28 days for every 1000 eyes treated with ocriplasmin (95% CI 16 more to 210 more).
Eyes receiving ocriplasmin were more likely to achieve 3‐line or greater improvement in BCVA at six months (RR 1.95, 95% CI 1.07 to 3.53; 674 eyes, 3 RCTs, moderate‐certainty evidence). Approximately 61/1000 eyes will have a 3‐line or greater improvement in BCVA at six months without treatment. An additional 58 eyes will have 3‐line or greater improvement in BCVA at six months for every 1000 eyes treated with ocriplasmin (95% CI 9 more to 154 more).
Receiving ocriplasmin also reduced the requirement for vitrectomy at six months (RR 0.67, 95% CI 0.50 to 0.91; 689 eyes, 3 RCTs, moderate‐certainty evidence). Approximately 265/1000 eyes will require vitrectomy at six months without treatment and 87 fewer eyes will require vitrectomy for every 1000 eyes treated with ocriplasmin (95% CI 24 fewer to 132 fewer).
Treatment with ocriplasmin resulted in a greater improvement in validated Visual Function Questionnaire form score at six months (mean improvement difference 2.7 points, 95% CI 0.8 to 4.6; 652 eyes, 2 RCTs, moderate‐certainty evidence).
Eyes receiving ocriplasmin were more likely to have an adverse event (RR 1.22, 95% CI 1.09 to 1.37, 909 eyes, 4 RCTs, moderate‐certainty evidence). Approximately 571/1000 eyes will have an adverse event with sham or placebo injection and 106 more eyes will have an adverse event for every 1000 eyes treated with ocriplasmin (95% CI 52 more to 212 more).
Authors' conclusions
Evidence from a limited number of RCTs suggests that ocriplasmin is useful in the treatment of sVMA. However, up to 20% of eyes treated with ocriplasmin will still require additional treatment with PPV within six months. There were more ocular adverse events in eyes treated with ocriplasmin than control (sham or placebo injection) treatment. Many of these adverse events, particularly vitreous floaters and photopsia, are known to be associated with posterior vitreous detachment. At present however, there is minimal published long‐term safety data on eyes treated with ocriplasmin. Further large RCTs comparing ocriplasmin with other management options for sVMA would be beneficial.
Plain language summary
Ocriplasmin for symptomatic vitreomacular adhesion
What is the aim of this review? The aim of this Cochrane Review was to find out how well ocriplasmin works in the treatment of symptomatic vitreomacular adhesion (sVMA). Cochrane Review authors collected and analysed all relevant studies to answer this question and found four studies.
Key messages People with sVMA treated with ocriplasmin have an increased chance of release of sVMA and improved vision compared with people who are not treated with ocriplasmin (high‐certainty evidence). They are also probably less likely to require surgery, but one in five people with sVMA treated with ocriplasmin will probably still require surgery at a later date to treat sVMA (moderate‐certainty evidence).
What was studied in the review? With age, the gel‐like substance (vitreous) that fills the eye begins to pull away from the back of the eye (retina). Sometimes the vitreous remains attached to the retina and causes damage to the retina as it pulls away, leading to visual loss. This is known as symptomatic vitreomacular adhesion or sVMA. sVMA includes two related conditions, vitreomacular traction and macular hole.
The standard treatment for sVMA is surgery. Ocriplasmin is an alternative, less invasive, treatment. This is an enzyme that can be injected directly into the eye to release the vitreous from the retina.
What are the main results of the review? Cochrane Review authors found four studies that compared ocriplasmin with control (sham or placebo treatment) for the treatment of sVMA. All four studies were sponsored by the manufacturers of ocriplasmin.
The review showed that:
• ocriplasmin increases the chance of sVMA resolution compared with no treatment (high‐certainty evidence); • people with sVMA treated with ocriplasmin have improved vision compared with people who are not treated with ocriplasmin (high‐certainty evidence); • treatment with ocriplasmin probably reduces the requirement for surgery, but approximately one in five people treated with ocriplasmin may require further surgery at a later date (moderate‐certainty evidence); • there were more ocular adverse events in eyes treated with ocriplasmin than control (sham or placebo injection) treatment.
How up‐to‐date is this review? Cochrane Review authors searched for studies that had been published up to 24 February 2017.
Summary of findings
Summary of findings for the main comparison. Ocriplasmin injection compared with control for symptomatic vitreomacular adhesion.
| Ocriplasmin injection compared with control for symptomatic vitreomacular adhesion | ||||||
|
Patient or population: people with symptomatic vitreomacular adhesion Settings: eye hospital Intervention: ocriplasmin injection Comparison: sham or placebo injection | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of eyes (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham or placebo injection | Ocriplasmin injection | |||||
|
Complete release of vitreous adhesion Follow‐up: 28 days |
97 per 1000 |
334 per 1000 (193 to 579) |
RR 3.46 (2.00 to 6.00) |
859 (4 studies) |
⊕⊕⊕⊕ High | ‐ |
|
Closure of macular hole Follow‐up: 28 days to 24 months |
123 per 1000 |
354 per 1000 (185 to 679) |
RR 2.87 (1.50 to 5.51) |
229 (3 studies) |
⊕⊕⊕⊕ High | ‐ |
|
Complete posterior vitreous detachment Follow‐up: 28 days |
40 per 1000 |
118 per 1000 (56 to 250) |
RR 2.94 (1.39 to 6.24) |
689 (3 studies) |
⊕⊕⊕⊕ High | ‐ |
|
3‐line or greater improvement in best‐corrected visual acuity Follow‐up: 6 months |
61 per 1000 | 119 per 1000 (70 to 215) |
RR 1.95 (1.07 to 3.53) |
674 (3 studies) |
⊕⊕⊕⊝ Moderatea |
‐ |
|
Requirement for vitrectomy Follow‐up: 6 months |
265 per 1000 |
178 per 1000 (133 to 241) |
RR 0.67 (0.50 to 0.91) |
689 (3 studies) |
⊕⊕⊕⊝ Moderatea |
‐ |
|
Mean change in validated visual function questionnaire score from baseline Score ranges from 0 to 100, higher scores are better visual function Follow‐up: 6 months |
Mean change in NEI‐VFQ score was 0.7 | NEI‐VFQ score was 2.7 higher (0.8 higher to 4.6 higher) | ‐ | 652 (2 studies) |
⊕⊕⊕⊝ Moderatea |
‐ |
|
Any ocular adverse event Follow‐up: 6 months |
571 per 1000 |
697 per 1000 (623 to 783) |
RR 1.22 (1.09 to 1.37) |
909 (4 studies) |
⊕⊕⊕⊝ Moderatea |
‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NEI‐VFQ: National Eye Institute Visual Function Questionnaire; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | ||||||
aDowngraded one level for imprecision (‐1).
Background
Description of the condition
In healthy eyes, the posterior vitreous face lies in contact with the internal limiting membrane (ILM) of the retina with various points of stronger adhesion such as the macula, vasculature and optic disc. Over time, the structure of the vitreous liquefies in a process known as synchysis, with reduction in the adhesive forces between vitreous and ILM. This often results in the vitreous gel detaching from all parts of the retina, except at the vitreous base anteriorly, in a normal process known as posterior vitreous detachment (PVD) (Foos 1982). The process usually starts with focal detachment in the perifovea of the superior quadrant and then extends slowly for years until eventually resulting in a complete PVD with release of vitreopapillary adhesion (Ito 2003; Johnson 2010; Uchino 2001). However, in certain cases, incomplete PVD may occur, leaving the vitreous in contact with the macula or optic disc, or both.
Although, anatomically, vitreomacular adhesion (VMA) may refer to a normal asymptomatic state, clinically, the term is used when VMA occurs in the context of an incomplete PVD. There is a spectrum of VMA associated with incomplete PVD, which ranges from asymptomatic, non‐tractional VMA to extensive distortion of the retinal structure due to vitreomacular traction (VMT) which may result in loss of visual function. These distinctions tend to be based on optical coherence tomography (OCT), sometimes in reference to defined photographic standards (Simpson 2012). However, it is important to note that the OCT changes, which may include retinal thickening and intraretinal oedema, do not always correlate with visual function and symptoms.
Symptomatic vitreomacular adhesion (sVMA) is defined as visual loss secondary to foveal damage caused by abnormal VMT. sVMA includes isolated VMT, impending macular hole (MH) and MH with persisting vitreous attachment (Jackson 2013a). Impending MH is often grouped with VMT. Epiretinal membrane (ERM) often coexists with sVMA. It is possible that VMA influences the clinical course of, or may be associated with, other diseases such as diabetic macular oedema, retinal vein occlusion or neovascular age‐related macular degeneration, although the data are sometimes conflicting (Jackson 2013a; Jackson 2013b; Nomura 2014; Simpson 2012; Terao 2014; Waldstein 2014; Yoon 2014). Whilst there may be an association between sVMA and these other diseases, it is not certain that this is causal (Simpson 2012). Consequently, it is difficult to define the prevalence of sVMA. One study reported that VMA may occur in isolation or in association with other eye disease in approximately 1.5% of the population (Jackson 2013a). However, the majority of these cases occurred alongside ERM, and thus the VMA may not be responsible for visual loss. Excluding cases associated with ERM reduced the prevalence to 0.35% in the same population‐based study; however, this figure also included cases with other diseases, such as wet age‐related macular degeneration and diabetic macular oedema (Jackson 2013a). If only cases of isolated VMA/VMT with or without MH were considered, then the prevalence of sVMA was 171.5 per 100,000 population (Jackson 2013a).
The natural history of sVMA varies. sVMA may spontaneously resolve, with detachment of the posterior vitreous face from the ILM (Steel 2013). One study of 53 eyes showed a complete PVD occurred in 11% of eyes over 60 months' follow‐up (Hikichi 1995). Weinard and colleagues reported that approximately 10% of cases of VMT syndrome resolve spontaneously (Weinard 2009). Other studies have found spontaneous resolution in 17% to 35% of cases with VMT (Almeida 2015; Theodossiadis 2014; Zhang 2015). Eyes with VMT and isolated inner retinal distortion, as well as those receiving vitreous injections, have an increased likelihood of VMT release (Almeida 2015). Poor prognostic indicators for spontaneous release include the presence of ERM and large horizontal adhesion diameter (Haller 2015; Jackson 2016; Theodossiadis 2014; Zhang 2015). It has been shown that many, if not most, MHs result from persistent VMT which either fully detaches from the retina causing an MH, or remains attached at the edge of the hole (Chauhan 2000; Gass 1988; Gaudric 1999; La Cour 2002; Tanner 2001).
Description of the intervention
Treatment strategies for VMA vary depending on disease severity. Asymptomatic VMT can be observed, since separation of the posterior vitreous face may occur spontaneously and without sequelae. However, a longer duration of VMT may lead to loss of vision and possibly lower efficacy of any subsequent intervention, and therefore treatment is often considered if symptoms are significant or visual acuity is reduced (Hikichi 1995; Melberg 1995; Sonmez 2008). If VMT progresses to MH then intervention is usually advised, and an evolving VMT/impending MH may likewise necessitate intervention.
If intervention is considered for sVMA, various strategies may be considered. Traditionally, pars plana vitrectomy (PPV) is the standard approach for VMT or MH (Steel 2013). Small uncontrolled studies reported that an intravitreal gas bubble can pneumatically release VMT, without the need for PPV, with success rates varying from 71% to 95% (Chan 1995; Mori 2007; Rodrigues 2013).
Pharmacological vitreolysis has been investigated as an alternative treatment for VMT, and for MH with persisting VMA (Benz 2010; De Smet 2009; Stalmans 2010; Stalmans 2012). Autologous plasmin, an enzyme that breaks down the laminin and fibronectin bonds maintaining vitreous adhesion, has been used perioperatively to induce a PVD during vitrectomy (Margherio 1998; Sakuma 2006; Williams 2001). However, autologous plasmin is not suited to the treatment of VMT due to its autolytic instability (Gandorfer 2008). Based on autologous plasmin, a recombinant DNA molecule, initially referred to as microplasmin, and more recently ocriplasmin (Jetrea; ThromboGenics, Leuven, Belgium), was developed to provide the same catalytic properties but with greater stability.
Ocriplasmin is administered as a single intravitreal injection of 125 μg in 0.1 mL. It has marketing authorisation for the treatment of VMT, including when associated with MH of diameter of 400 μm or less (SmPC 2013). In the UK, the National Institute for Health and Care Excellence (NICE) supports the use of ocriplasmin for adults with VMT causing severe sight problems or a macula hole up to 400 μm, in the absence of ERM (NICE 2013).
How the intervention might work
Ocriplasmin is a proteolytic enzyme which targets laminin and fibronectin, both of which are important structural components of the interface between the vitreous and the retina. It is a truncated form of the human serine protease plasmin which functions in a two‐stage mechanism; liquefaction of the vitreous and vitreoretinal separation (Kuppermann 2012).
Why it is important to do this review
Ocriplasmin has marketing authorisation in Europe and the USA and is the only licensed, non‐surgical treatment for sVMA. MH is the second most common indication for PPV, and both MH and VMT can cause substantial visual problems (Jackson 2013c). This review is important as it assessed the efficacy and safety of ocriplasmin treatment.
Objectives
To assess the efficacy and safety of ocriplasmin compared to no treatment, sham or placebo for the treatment of sVMA.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) only.
Types of participants
We included participants with a diagnosis of sVMA, including VMT and MH of 400 μm or less with persisting VMA. There were no restrictions with regards to gender, age or ethnicity.
Types of interventions
We included any RCT in which intravitreal ocriplasmin was compared to no treatment, sham injection or placebo.
Types of outcome measures
Primary outcomes
Proportion of eyes with complete release of vitreous adhesion as determined by analysis of OCT images captured 28 days after ocriplasmin, sham or placebo treatment.
Secondary outcomes
Proportion of eyes with closure of MH as determined by analysis of OCT images captured 28 days after ocriplasmin, sham or placebo treatment.
Proportion of eyes with complete PVD as measured by clinical examination or B‐scan ultrasonography 28 days after ocriplasmin, sham or placebo treatment.
Proportion of eyes with 3‐line or greater improvement in best corrected visual acuity (BCVA) from baseline, measured using Early Treatment Diabetic Retinopathy Study (ETDRS) at 4 m or Snellen chart, at six months after ocriplasmin, sham or placebo treatment.
Proportion of eyes requiring PPV within six months of ocriplasmin, sham or placebo treatment (as recommended by the investigator if the underlying condition deteriorated, BCVA worsened by more than 2 lines on ETDRS or Snellen chart, or if the underlying condition had not improved within 28 days after treatment).
Mean change in validated Visual Function Questionnaire (VFQ) score from baseline, measured at six months after ocriplasmin, sham or placebo treatment.
Safety outcomes
Description of ocular adverse events and serious adverse events, and any non‐ocular serious adverse events attributed to ocriplasmin or no treatment/sham/placebo.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for randomised controlled trial and controlled clinical trials. There were no language or publication year restrictions. The date of the search was 24 February 2017.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 24 February 2017) (Appendix 1);
MEDLINE Ovid (1946 to 24 February 2017) (Appendix 2);
Embase Ovid (1980 to 24 February 2017) (Appendix 3);
PubMed (1946 to 24 February 2017) (Appendix 4);
ISRCTN registry (www.isrctn.com/editAdvancedSearch; searched 24 February 2017) (Appendix 5);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 24 February 2017) (Appendix 6);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 24 February 2017) (Appendix 7).
Searching other resources
We searched the reference lists of included studies for other possible studies. We did not search proceedings from conferences specifically, because such RCTs presented at these meetings were searched by Cochrane Eyes and Vision and included in CENTRAL.
Data collection and analysis
Selection of studies
Three authors (JN, VK and TJ) independently assessed the results identified by the searches and classified each record as either possibly relevant or definitely not relevant. We then obtained full‐text copies of all possibly relevant records, and three authors (JN, VK and TJ) classified them as definitely include, unsure or definitely exclude based on the criteria for inclusion. In the event of any difficulty in classification due to lack of clarity or data, we contacted study investigators for further information. All contacted authors responded to our requests. We resolved discrepancies by consensus following discussion between authors (JN, VK and TJ) and documented this in the review. All excluded records were documented.
Data extraction and management
Two authors (JN and VK) independently extracted trial data for the primary and secondary outcomes onto paper data extraction forms developed by Cochrane Eyes and Vision. Subsequently, data were transcribed into Review Manager 5 (RevMan 2014) by one author (JN) and verified by a second author (VK). Any discrepancies were resolved by consensus between authors (JN, VK and TJ) and documented in the review.
We collected the following information on study characteristics (see Appendix 8):
study design: parallel group RCT/within‐person RCT/one or both eyes reported;
participants: country, total number of participants, age, sex, inclusion and exclusion criteria;
intervention and comparator details: including number of people (eyes) randomised to each group;
primary and secondary outcomes as measured and reported in the trials, adverse events;
length of follow‐up;
date study conducted;
funding and conflicts of interest.
We extracted the following data from each included study for intervention and comparator groups separately:
number of events and number of participants for outcome data collected for dichotomous variables (release of vitreous adhesion at 28 days, closure of MH at 28 days and complete PVD at 28 days);
mean, standard deviation and number of participants for outcome data measured for continuous variables (change in BCVA at six months and change in validated VFQ at six months). To compare visual acuity across studies, the mean BCVA was converted to logarithm of the minimum angle of resolution units (logMAR). Counting fingers vision was assigned a logMAR acuity of 1.6, hand movements 1.9, light perception 2.2 and no light perception 2.5 (Westheimer 1979). The default VFQ assessed was the National Eye Institute Visual Functioning Questionnaire ‐ 25 (NEI‐VFQ25).
We collected evidence of harm from RCTs only.
Assessment of risk of bias in included studies
Two authors (JN and VK) independently assessed the included trials for bias using the methods and grades described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We assessed the following: methods of sequence generation used for randomisation; allocation concealment; masking (blinding) of outcome assessors; masking of participants and personnel; incomplete outcome data; selective outcome reporting; other bias. We considered the use, or not, of independent masked OCT image analysis assessors in the assessment of bias. We then classified each item as 'low,' 'high' or 'unclear' risk of bias.
Measures of treatment effect
We presented dichotomous data as risk ratios (RR) with 95% confidence intervals (CI);
-
Primary outcome:
resolution of VMA.
-
Secondary outcomes:
closure of MH;
complete PVD;
proportion of eyes with 3‐line or greater gain in BCVA;
requirement for PPV.
We presented continuous data as mean differences with 95% CIs:
change in validated VFQ measure.
Unit of analysis issues
Trials randomised one or both eyes to the intervention or comparator. If people were randomly allocated to treatment but only one eye per person was included in the trial then there was no unit of analysis issue. In these cases, we documented how the eye was selected and if this was done before randomisation. If people were randomly allocated to treatment but both eyes were included and reported, we planned to analyse as 'clustered data,' that is, adjust for within‐person correlation. If the study was a within‐person study, that is, one eye was randomly allocated to intervention and the other eye received the comparator, then we planned to analyse as paired data. We planned to contact the trial investigators for further information to do this if necessary.
Dealing with missing data
In the event of missing trial outcome data, we contacted the authors of the trial to understand why the data were missing. If no response was received within four weeks, we used the information provided in the published articles. Missing data were handled in accordance with the guidelines given in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We planned to perform sensitivity analyses on the impact of missing data and comment on the findings in the discussion of the review.
Assessment of heterogeneity
We assessed heterogeneity and inconsistency among trials statistically using an I2 value (> 50%) to assess if variability in effect was due to sampling error. We also planned to assess diversity among studies by reviewing participant characteristics and trial methodology.
Assessment of reporting biases
We assessed selective outcome reporting by comparing intended outcomes in published protocols, published methods papers and clinical trial registries to reported outcomes in the results sections of trial reports. If there were 10 or more eligible RCTs, we planned to use a funnel plot to assess for study‐reporting bias.
Data synthesis
If there were three or fewer eligible RCTs then we planned to use a fixed‐effect model for the meta‐analyses. If there were more than three included trials, we planned to use a random‐effects model instead. If we had evidence of high heterogeneity (e.g. I2 > 50%), it would not be sensible to pool the data from different trials; in which case, we planned to do a narrative summary of the results.
Subgroup analysis and investigation of heterogeneity
If trials demonstrated clinical heterogeneity and sufficient data were available, including age (< 65 years, 65 years and over), presence of ERM, size of adhesion (less than 1500 μm, 1500 μm or greater) and sVMA subtype (isolated VMT, and MH with persisting vitreous attachment), we planned to perform subgroup analyses for the primary outcome.
Sensitivity analysis
We planned to conduct one sensitivity analysis, excluding studies that were at high risk of bias in one or more domains.
'Summary of findings' table
We prepared a 'Summary of findings' table for the following outcomes:
resolution of VMA at 28 days;
complete PVD at 28 days;
closure of MH at 28 days;
proportion gaining 3‐line or greater improvement in BCVA at six months;
requirement of PPV at six months;
change in validated VFQ measure at six months;
adverse and serious adverse events.
Two authors (JN and VK) independently graded the overall certainty of the evidence for each outcome using the GRADE Working Group classification (GRADEpro 2014).
Results
Description of studies
Results of the search
The electronic searches yielded 418 records (Figure 1). The Cochrane Information Specialist scanned the search results, removed 136 duplicates and then removed 123 references which were irrelevant to the scope of the review. We screened the remaining 159 reports and obtained 14 full‐text reports for further assessment. We included five reports of four RCTs, three reports (Haller 2015; Stalmans 2012; Varma 2015) analysed separate outcomes from the same two RCTs (TG‐MV‐006 2012; TG‐MV‐007 2012). We excluded nine reports of nine studies (see Characteristics of excluded studies for details). We did not identify any ongoing studies from our searches of clinical trials registries.
1.
Study flow diagram.
Included studies
The following is a summary of the characteristics of the four RCTs that met the review inclusion criteria (MIVI‐IIT 2010; OASIS 2016; TG‐MV‐006 2012; TG‐MV‐007 2012). All data were initially obtained from published literature, then verified for discrepancies using the clinical trials registries described in the Methods section. See the Characteristics of included studies table for further information.
Types of participants
The four RCTs included enrolled 932 participants (932 eyes). All participants received individually randomised, parallel group treatment to a single eye. The age range of all included participants was 18 to 97 years. All included participants had evidence of focal VMA on OCT, BCVA of 20/25 or worse in the study eye and 20/400 or better in the fellow eye (ETDRS acuity chart). Exclusion criteria were: active proliferative diabetic retinopathy, high myopia (axial length greater than 26 mm or more than ‐8 dioptres), previous vitrectomy or uncontrolled glaucoma, previous intravitreal injections within the past three months in the study eye, intraocular surgery or laser photocoagulation within the past three months in the study eye or rhegmatogenous retinal detachment in either eye. Additional exclusion criteria in TG‐MV‐007 2012 were: neovascular age‐related macular degeneration, retinal vascular occlusion, aphakia, MH greater than 400 μm in diameter, vitreous opacification or lenticular or zonular instability. In OASIS 2016, eyes with an ERM were also excluded from enrolment.
Types of interventions
MIVI‐IIT 2010 compared a single injection of ocriplasmin 75 μg, ocriplasmin 125 μg or ocriplasmin 175 μg with sham injection (conjunctiva touched with a blunt needle to simulate an injection) to establish the optimal dose. A fourth cohort of participants underwent an initial injection of ocriplasmin 125 μg, but also a repeat injection at four and eight weeks if VMA was still present on OCT. Therefore, only data from participants receiving ocriplasmin 125 μg in this study were extracted and pooled for analysis. TG‐MV‐006 2012 and TG‐MV‐007 2012 both compared a single injection of ocriplasmin 125 μg with placebo injection (of the same vehicle used in the ocriplasmin injection). OASIS 2016 compared a single injection of ocriplasmin 125 μg with sham injection (syringe hub pressed into conjunctiva to simulate an injection).
Types of outcome measures
All four studies reported data for some of our primary and secondary outcome measures. No trial reported data for every outcome measure. Two trial reports (OASIS 2016; Varma 2015) provided data on participant‐reported outcome measures using the NEI‐VFQ25.
Data synthesis, subgroup and sensitivity analyses
As the search identified four trials, we used a random‐effects model (see Data synthesis). As there was no evidence of significant heterogeneity for the primary outcomes (I2 < 50%), we pooled data and performed no subgroup analyses of the primary outcome. Since no studies had a high risk of bias in any domain, we did not conduct a sensitivity analysis.
Excluded studies
We excluded nine articles after reviewing full‐text copies (Benz 2010; De Smet 2009; Dugel 2015; Elbendary 2011; Lanzetta 2014a; Lanzetta 2014b; Lescrauwaet 2016; Jackson 2017; Novack 2015). See Characteristics of excluded studies table for details.
Risk of bias in included studies
2.
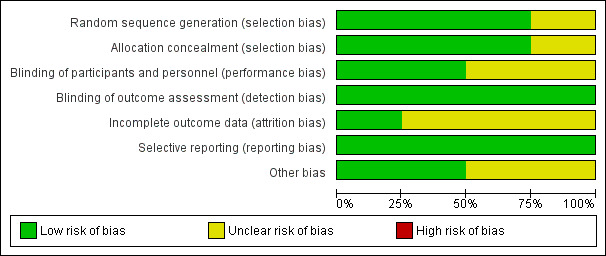
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
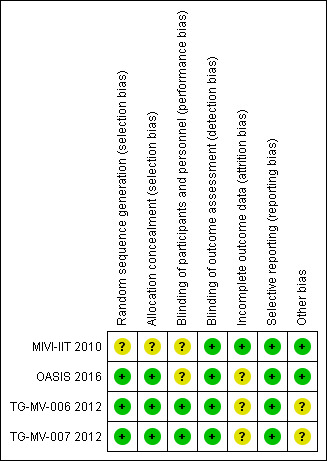
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
MIVI‐IIT 2010 did not describe the method of sequence generation, and provided insufficient information to also assess allocation concealment (Stalmans 2010). TG‐MV‐006 2012 and TG‐MV‐007 2012 clearly described randomisation and allocation concealment, which as a centralised telephone‐based system with blocks of treatment assigned to sites (Haller 2015; Stalmans 2012; Varma 2015). OASIS 2016 clearly described the method of randomisation, which used a centralised interactive voice response system.
Blinding
Two trials adequately masked participants and investigators (TG‐MV‐006 2012; TG‐MV‐007 2012). However, two trials did not mask investigators to sham injections (MIVI‐IIT 2010; OASIS 2016), which may have induced a different sensation to a true injection. The risk of performance bias was graded as unclear for both studies.
Incomplete outcome data
We graded risk of bias as low in one study (MIVI‐IIT 2010), and unclear in the other three studies (OASIS 2016; TG‐MV‐006 2012; TG‐MV‐007 2012). Unclear risk was due to losses to follow‐up not being reported and being unequal in different study groups. In addition, OASIS 2016 randomised 200 participants, but 50 participants were later found to be incorrectly enrolled by the central reading centre for a variety of reasons including MH greater than 400 μm, presence of ERM or no VMA at baseline. A subgroup analysis of this smaller cohort of participants, who met the inclusion and exclusion criteria, was performed, but only on outcome data for VMA release.
One trial reported a dilution error, which resulted in an extra participant treated in the ocriplasmin 125 μg cohort and one less participant in the ocriplasmin 175 μg cohort (MIVI‐IIT 2010).
Selective reporting
All studies reported on all prespecified primary and secondary outcomes (MIVI‐IIT 2010; OASIS 2016; TG‐MV‐006 2012; TG‐MV‐007 2012).
Other potential sources of bias
Two studies reported a baseline imbalance between study groups as pseudophakia was more common in the ocriplasmin group than in the placebo group and there were more women in the ocriplasmin group than in the placebo group (TG‐MV‐006 2012; TG‐MV‐007 2012). Therefore, this was at unclear risk of bias.
Effects of interventions
See: Table 1
See Table 1.
1. Proportion of eyes with complete release of vitreous adhesion
All four RCTs provided data for proportion of eyes with complete release of vitreous adhesion as determined by analysis of OCT images captured 28 days after ocriplasmin, sham or placebo treatment (MIVI‐IIT 2010; OASIS 2016; TG‐MV‐006 2012; TG‐MV‐007 2012). After excluding participants with protocol violations from OASIS 2016, analysis of the pooled data showed higher complete release of vitreous adhesion in the ocriplasmin group compared with control (placebo or sham) treatment (RR 3.46, 95% CI 2.00 to 6.00; 859 eyes; 4 studies; high‐certainty evidence; Analysis 1.1). A total of 97/1000 eyes had VMA release within 28 days without treatment. An additional 237 eyes had VMA release within 28 days for every 1000 eyes treated with ocriplasmin (95% CI 96 more to 482 more).
1.1. Analysis.
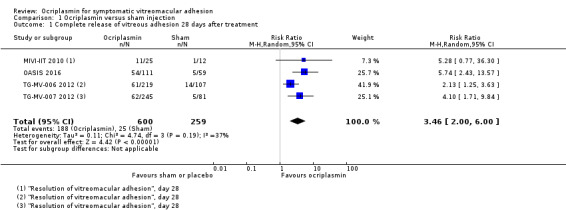
Comparison 1 Ocriplasmin versus sham injection, Outcome 1 Complete release of vitreous adhesion 28 days after treatment.
2. Proportion of eyes with closure of macular hole
Three studies provided data for proportion of eyes with closure of MH as determined by analysis of OCT images captured 28 days after ocriplasmin, sham or placebo treatment (OASIS 2016; TG‐MV‐006 2012; TG‐MV‐007 2012); data from MIVI‐IIT 2010 could not be included in this analysis as the original paper did not provide a breakdown of the ocriplasmin doses used to treat MH. OASIS 2016 measured MH closure at three months and the closure rate remained the same to the end of the study at 24 months. After excluding 14 participants incorrectly enrolled in OASIS 2016 due to MH being greater than 400 μm, analysis of the pooled data showed higher closure of MH in the ocriplasmin group compared with control (placebo or sham) treatment (RR 2.87, 95% CI 1.50 to 5.51; 229 eyes; 3 studies; high‐certainty evidence; Analysis 1.2). A total of 123/1000 eyes with MHs had closure with no treatment. An additional 231 eyes had MH closure for every 1000 eyes treated with ocriplasmin (95% CI 62 more to 556 more).
1.2. Analysis.
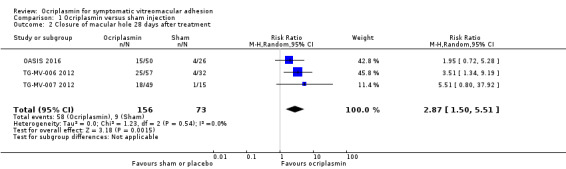
Comparison 1 Ocriplasmin versus sham injection, Outcome 2 Closure of macular hole 28 days after treatment.
3. Proportion of eyes with complete posterior vitreous detachment
Three studies provided data for proportion of eyes with complete PVD as measured by clinical examination or B‐scan ultrasonography 28 days after ocriplasmin, sham or placebo treatment (MIVI‐IIT 2010; TG‐MV‐006 2012; TG‐MV‐007 2012). Analysis revealed a higher incidence of complete PVD at 28 days in eyes treated with ocriplasmin compared with control (placebo or sham) treatment (RR 2.94, 95% CI 1.39 to 6.24; 689 eyes; 3 studies; high‐certainty evidence; Analysis 1.3). A total of 40/1000 eyes had complete PVD within 28 days without treatment. An additional 78 eyes had complete PVD within 28 days for every 1000 eyes treated with ocriplasmin (95% CI 16 more to 210 more).
1.3. Analysis.
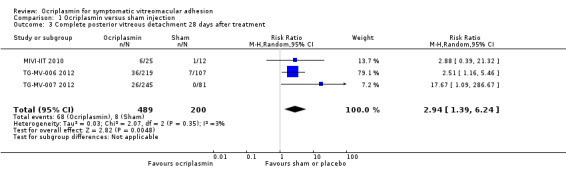
Comparison 1 Ocriplasmin versus sham injection, Outcome 3 Complete posterior vitreous detachment 28 days after treatment.
4. Proportion of eyes with 3‐line or greater improvement in best corrected visual acuity
Three studies provided data for proportion of eyes with 3‐line or greater improvement in BCVA measured using the ETDRS scale, at six months after ocriplasmin, sham or placebo treatment (MIVI‐IIT 2010; TG‐MV‐006 2012; TG‐MV‐007 2012). Due to separate outcomes reported for eyes with and without full‐thickness MH, and large numbers of participants not meeting eligibility criteria, data were not included from OASIS 2016. Eyes that had undergone PPV in MIVI‐IIT 2010 during this six‐month period were also excluded. Analysis of the pooled data revealed that eyes treated with ocriplasmin without PPV were more likely to achieve 3‐line or greater improvement in BCVA than control (sham or placebo) eyes (RR 1.95, 95% CI 1.07 to 3.53; 674 eyes; 3 studies; moderate‐certainty evidence; Analysis 1.4). A total of 61/1000 eyes had 3‐line or greater improvement in BCVA at six months without treatment. An additional 58 eyes had 3‐line or greater improvement in BCVA at six months for every 1000 eyes treated with ocriplasmin (95% CI 9 more to 154 more).
1.4. Analysis.
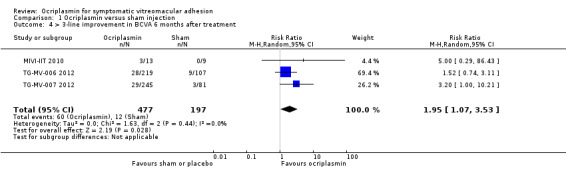
Comparison 1 Ocriplasmin versus sham injection, Outcome 4 > 3‐line improvement in BCVA 6 months after treatment.
5. Proportion of eyes requiring vitrectomy within six months of ocriplasmin, sham or placebo treatment
Three studies provided data for proportion of eyes requiring vitrectomy (MIVI‐IIT 2010; TG‐MV‐006 2012; TG‐MV‐007 2012). All three RCTs defined the requirement for vitrectomy as "recommended by the investigator if the underlying condition deteriorated, BCVA worsened by more than two lines on ETDRS or Snellen chart, or if the underlying condition had not improved within 28 days after treatment." Due to separate outcomes reported for eyes with and without full‐thickness MH, and large numbers of participants not meeting eligibility criteria, data were not included from OASIS 2016. Analysis revealed a lower requirement for vitrectomy in eyes treated with ocriplasmin compared with control (placebo or sham) treatment (RR 0.67, 95% CI 0.50 to 0.91; 689 eyes; 3 studies; moderate‐certainty evidence; Analysis 1.5). A total of 265/1000 eyes required vitrectomy at six months without treatment and 87 fewer eyes required vitrectomy for every 1000 eyes treated with ocriplasmin (95% CI 24 fewer to 132 fewer).
1.5. Analysis.
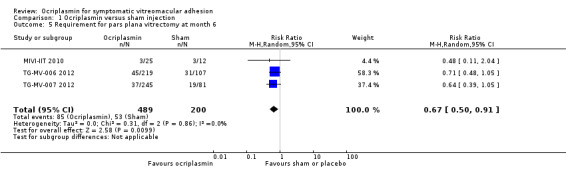
Comparison 1 Ocriplasmin versus sham injection, Outcome 5 Requirement for pars plana vitrectomy at month 6.
6. Mean change in validated Visual Function Questionnaire score from baseline measured at six months after ocriplasmin, sham or placebo treatment
One trial reported data for mean change in validated VFQ score from baseline (Varma 2015), which analysed pooled participant‐reported visual function outcomes for TG‐MV‐006 2012 and TG‐MV‐007 2012. In all eyes across both studies, mean increases in the composite NEI‐VFQ25 score at six months from baseline were greater in eyes treated with ocriplasmin (464 eyes) than placebo (188 eyes) (mean change: 3.4 with ocriplasmin versus 0.7 with placebo; P = 0.005). We calculated the mean difference as 2.7 (95% CI 0.8 to 4.6). Visual function data was also reported in OASIS 2016, but this was not reported for the subgroup who met the inclusion and exclusion criteria following central reading centre analysis.
7. Adverse effects
Due to inconsistencies between the studies and differences in control groups (placebo injection versus sham injection), we did not perform a pooled analysis of adverse events. Instead, a descriptive account of the types of ocular adverse event is provided below, based on data from three studies (OASIS 2016; TG‐MV‐006 2012; TG‐MV‐007 2012). Although a large number of participants were incorrectly enrolled in OASIS 2016, safety data are presented for all participants who underwent intervention with ocriplasmin or control treatment.
7.1. Any ocular adverse events
These were defined as any ocular adverse event that did not meet the criteria for a serious ocular adverse event (see '7.2. Any serious ocular adverse events'). All four RCTs provided data for any ocular adverse event (MIVI‐IIT 2010; OASIS 2016; TG‐MV‐006 2012; TG‐MV‐007 2012). Analysis revealed more ocular adverse events in eyes treated with ocriplasmin compared with placebo or sham‐treated eyes (RR 1.22, 95% CI 1.09 to 1.37; 909 eyes; 4 studies; moderate‐certainty evidence; Analysis 1.6).
1.6. Analysis.
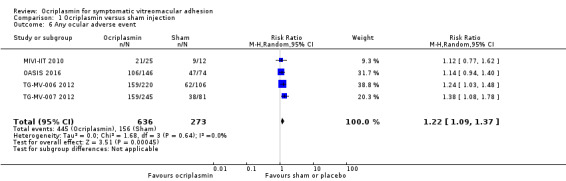
Comparison 1 Ocriplasmin versus sham injection, Outcome 6 Any ocular adverse event.
A breakdown of the most frequently reported ocular adverse events is listed in the table below (n = number of eyes affected, not total number of events). The first five ocular adverse events were participant‐reported. The most commonly reported ocular adverse events following ocriplasmin treatment were vitreous floaters (affecting 133/611 eyes or 21.8%), photopsia (affecting 98/611 eyes or 16.0%) and injection‐related eye pain (affecting 83/611 eyes or 13.6%). The incidence of vitreous floaters, photopsia, injection‐related eye pain, blurred vision and visual impairment was significantly greater in eyes treated with ocriplasmin than those treated with sham or placebo injection.
| Study | MIVI‐IIT 2010 | TG‐MV‐006 2012 | TG‐MV‐007 2012 | OASIS 2016 | ||||
|
Ocriplasmin (n = 25) |
Control (n = 12) |
Ocriplasmin (n = 220) |
Control (n = 106) |
Ocriplasmin (n = 245) |
Control (n = 81) |
Ocriplasmin (n = 146) |
Control (n = 74) |
|
| Any ocular adverse event | 21 | 9 | 159 | 62 | 159 | 38 | 106 | 47 |
| Vitreous floatersa | ‐ | ‐ | 42 | 9 | 36 | 5 | 55 | 6 |
| Photopsiaa | ‐ | ‐ | 36 | 4 | 19 | 1 | 43 | 5 |
| Injection‐related eye paina | ‐ | ‐ | 33 | 6 | 30 | 5 | 20 | 6 |
| Blurred visiona | ‐ | ‐ | 24 | 4 | 16 | 2 | 27 | 4 |
| Visual impairmenta | ‐ | ‐ | 21 | 3 | 4 | 0 | 21 | 4 |
| Conjunctival haemorrhage | 8 | 3 | 34 | 14 | 34 | 10 | 14 | 1 |
| Increased intraocular pressurea | ‐ | ‐ | 9 | 10 | 9 | 0 | 10 | 10 |
| Retinal teara | ‐ | ‐ | 5 | 2 | 1 | 3 | 2 | 5 |
| Cataracta | ‐ | ‐ | 14 | 12 | 12 | 5 | 19 | 10 |
| Anterior chamber cellsb | 1 | 0 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Iridocyclitisb | 1 | 0 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Vitritisb | 3 | 0 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
aOcular adverse events not reported in MIVI‐IIT.
bOcular adverse events not reported in OASIS 2016, TG‐MV‐006 2012, or TG‐MV‐007 2012.
Note: the control group in MIVI‐IIT 2010 and OASIS 2016 was sham injection. The control group in TG‐MV‐007 2012 and TG‐MV‐006 2012 was placebo injection.
7.2. Any serious ocular adverse events
Two studies defined serious ocular adverse event as: an event resulting in persistent or clinically significant disability, incapacity or both; an event requiring inpatient hospitalisation or prolongation of an existing hospital stay; or an event that was considered to be medically important (TG‐MV‐006 2012; TG‐MV‐007 2012). One study did not provide a definition of a serious ocular adverse event (OASIS 2016). MIVI‐IIT 2010 reported no instances of serious ocular adverse events.
A breakdown of the most frequently reported serious ocular adverse events is listed in the table below (n = number of eyes affected, not total number of events). The total incidence of serious ocular adverse events was 66/611 (10.8%) in eyes treated with ocriplasmin compared with 35/261 (13.4%) treated with sham or placebo injection. Most frequently reported was an increased or new macular hole, which occurred in 47/611 (7.7%) of eyes treated with ocriplasmin compared with 26/261 (9.9%) of eyes treated with sham or placebo injection. None of the included studies reported any cases of endophthalmitis.
| Study | MIVI‐IIT 2010 | TG‐MV‐006 2012 | TG‐MV‐007 2012 | OASIS 2016 | ||||
|
Ocriplasmin (n = 25) |
Control (n = 12) |
Ocriplasmin (n = 220) |
Control (n = 106) |
Ocriplasmin (n = 245) |
Control (n = 81) |
Ocriplasmin (n = 146) |
Control (n = 74) |
|
| Any serious ocular adverse event | 0 | 0 | 21 | 11 | 15 | 9 | 30 | 15 |
| Macular hole (increased or new) | ‐ | ‐ | 15 | 11 | 9 | 5 | 23 | 10 |
| Retinal detachment | ‐ | ‐ | 2 | 2 | 0 | 1 | 1 | 1 |
| Reduced visual acuity | ‐ | ‐ | 1 | 0 | 2 | 1 | 18 | 18 |
| Endophthalmitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Note: the control group in MIVI‐IIT 2010 and OASIS 2016 was sham injection. The control group in TG‐MV‐007 2012 and TG‐MV‐006 2012 was placebo injection.
Discussion
Summary of main results
We identified four RCTs, with 932 eyes, comparing ocriplasmin with control (placebo or sham injection) treatment. On full‐text analysis, we excluded 50 participants due to breaches of our inclusion criteria, and 23 participants because they received a different dose of ocriplasmin, giving 859 eyes for outcome analysis. The studies were conducted in Europe and the USA. We found that treatment with ocriplasmin increased the likelihood of complete release of vitreous traction compared to control (sham or placebo injection) treatment. Ocriplasmin was also associated with a 3‐line or greater improvement in BCVA and improvement in participant‐reported visual function.
There were however, more ocular adverse events in eyes treated with ocriplasmin than control (placebo or sham injection) treatment. Many of these adverse events, particularly vitreous floaters and photopsia, are known to be associated with posterior vitreous detachment. Of the serious ocular adverse events, increased or new macular hole was the most frequently reported. Given the high incidence in all eyes regardless of treatment, this most likely represents the natural history of VMT in a significant proportion of patients.
Overall completeness and applicability of evidence
Three of the included studies were large and contributed the majority of included participants (834) for our analysis (OASIS 2016; TG‐MV‐006 2012; TG‐MV‐007 2012). The other study, designed to determine the appropriate dose, contributed a relatively small number (25) of participants (MIVI‐IIT 2010). The control groups in the trials also varied, with participants in TG‐MV‐006 2012 and TG‐MV‐007 2012 receiving a placebo injection, and participants in MIVI‐IIT 2010 and OASIS 2016 receiving a sham injection. Due to the mechanical nature of the primary outcome, the variation in control group intervention could impact on the validity of the results, particularly adverse events. All four trials reported the same primary outcome and follow‐up periods were identical. One trial reported additional secondary outcome data at 24‐months (OASIS 2016).
It is important to note that OASIS 2016 initially randomised and treated 220 participants, but subsequent central reading centre analysis revealed 50 participants were ineligible due to lack of sVMA, presence of ERM or presence of MH greater than 400 μm. To comply with the inclusion and exclusion criteria of this review, we used only data from this smaller, central reading centre verified cohort of participants. Despite this attrition bias, sufficient pooled data were available, hence the impact of this bias was deemed small.
Quality of the evidence
Generally, we graded the risk of bias as low. However, two studies reported cases that did not complete the study on the ClinicalTrials.gov database (see Characteristics of included studies table) but the publications did not describe these losses to follow‐up (TG‐MV‐006 2012; TG‐MV‐007 2012). The authors confirmed using the last‐observation‐carried‐forward (LOCF) method for their missing outcome data, assuming the outcome was unlikely to change after discontinuation of treatment and likely to improve spontaneously over time. As these losses to follow‐up were not described in the original papers, we judged the risk of bias for incomplete outcome data as unclear.
Potential biases in the review process
We followed a standard Cochrane protocol (Neffendorf 2015), to minimise potential methodological biases in the review process.
Agreements and disagreements with other studies or reviews
In the UK, NICE recommends the use of ocriplasmin for adults with VMT causing severe sight problems or a MH up to 400 μm, in the absence of ERM. Our findings support this.
Subsequent publications and postmarket surveillance studies have addressed the safety of ocriplasmin. One large postmarket surveillance study found lower rates of adverse events than were reported in the registration studies, but noted that under‐reporting is common in post‐market surveillance studies (Hahn 2015). Members of the British and Eire Association of VitreoRetinal Surgeons (BEAVRS) have reported their experience with ocriplasmin in comparison to the MIVI‐TRUST trial data (Haynes 2017). They found a lower rate of MH closure and increased incidence of adverse events with ocriplasmin compared to the registration studies, but there is an uncertain risk of reporting bias.
Our review found a higher rate of vitreous floaters and photopsia with ocriplasmin, but no increased risk of loss in visual acuity and retinal detachment. There have been reports of acute reduction in visual acuity, electroretinography changes, dyschromatopsia, phacodonesis and OCT ellipsoid zone alteration, but the majority have been transient (Khan 2016; Neffendorf 2016).
Various studies and reviews have suggested certain subgroups of sVMA participants may be more likely to respond successfully to ocriplasmin treatment based on baseline characteristics such as adhesion diameter, lack of coexisting ERM, and the angle between the posterior vitreous cortex and the ILM (Haller 2015; Jackson 2016; Paul 2017). However, such analyses are exploratory, and without confirmatory prospective RCTs they are beyond the scope of this review.
There are different approaches to potentially manage sVMA including PPV, intravitreal gas injection, ocriplasmin and observation. Further research, ideally in a head‐to‐head trial, would be beneficial.
Authors' conclusions
Implications for practice.
We found evidence to support the use of ocriplasmin for the treatment of symptomatic vitreomacular adhesion (sVMA), although the number of studies was low. There are reported concerns about the safety of ocriplasmin treatment and there is debate within the vitreoretinal community regarding the advantages and disadvantages of ocriplasmin.
Implications for research.
Further large randomised controlled trials would augment our current understanding of the safety and efficacy of ocriplasmin. Ideally these would compare ocriplasmin with other commonly used management options, in particular observation or pars plana vitrectomy. Randomised controlled trials recruiting participants with baseline characteristics thought to improve the efficacy of ocriplasmin are warranted.
Acknowledgements
We acknowledge Cochrane Eyes and Vision for creating and executing the electronic search strategies. We thank David Steel and Jennifer Evans for their comments on the protocol and review, Catey Bunce for her comments on the review and Ana Quartilho for her comments on the protocol. We thank Anupa Shah for her assistance throughout the editorial process.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Vitreous Body] this term only #2 MeSH descriptor: [Vitreous Detachment] this term only #3 MeSH descriptor: [Retinal Perforations] this term only #4 MeSH descriptor: [Tissue Adhesions] this term only #5 vitreomacular near/3 (adhesion* or traction*) #6 VMA* or VMT* #7 macula* near/2 hole* #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 MeSH descriptor: [Fibrinolysin] this term only #10 MeSH descriptor: [Fibrinolytic Agents] this term only #11 MeSH descriptor: [Proteolysis] this term only #12 MeSH descriptor: [Peptide Fragments] this term only #13 ocriplasmin* or Jetrea* or Microplasmin* #14 #9 or #10 or #11 or #12 or #13 #15 #8 and #14
Appendix 2. MEDLINE Ovid search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. Vitreous Body/ 14. Vitreous Detachment/ 15. Retinal Perforations/ 16. Tissue Adhesions/ 17. (vitreomacular adj3 (adhesion$ or traction$)).tw. 18. (VMA$ or VMT$).tw. 19. (macula$ adj2 hole$).tw. 20. or/13‐19 21. Fibrinolysin/ 22. Fibrinolytic Agents/ 23. Proteolysis/ 24. Peptide Fragments/ 25. (ocriplasmin$ or Jetrea$ or Microplasmin$).tw. 26. or/21‐25 27. 20 and 26 28. 12 and 27
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase Ovid search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. vitreous body detachment/ 34. vitreous disease/ 35. retina tear/ 36. tissue adhesion/ 37. (vitreomacular adj3 (adhesion$ or traction$)).tw. 38. (VMA$ or VMT$).tw. 39. (macula$ adj2 hole$).tw. 40. or/33‐39 41. ocriplasmin/ 42. fibrinolytic agent/ 43. peptide fragment/ 44. (ocriplasmin$ or Jetrea$ or Microplasmin$).tw. 45. or/41‐44 46. 40 and 45 47. 32 and 46
Appendix 4. PubMed search strategy
(((vitreous body[MeSH Terms]) OR (vitreous detachment[MeSH Terms]) OR (Retinal Perforations[MeSH Terms]) OR (tissue adhesions[MeSH Terms]) OR (vitreomacular adhesion*[Text Word]) OR (vitreomacular traction*[Text Word]) OR (VMA*[Text Word] OR VMT*[Text Word]) OR (macula* AND hole*[Text Word])) AND ((fibrinolysin[MeSH Terms]) OR (fibrinolytic agents[MeSH Terms]) OR (proteolysis[MeSH Terms]) OR (peptide fragments[MeSH Terms]) OR (ocriplasmin*[Text Word] OR Jetrea*[Text Word] OR Microplasmin*[Text Word]))) AND (((randomized controlled trial[Publication Type]) OR (controlled clinical trial[Publication Type]) OR (random*[Text Word] OR placebo*[Text Word] OR trial*[Text Word] OR group*[Text Word])) AND (Medline[sb]))
Appendix 5. ISRCTN search strategy
Ocriplasmin OR Jetrea OR Microplasmin
Appendix 6. ClinicalTrials.gov search strategy
(vitreomacular adhesion OR vitreomacular traction OR macular hole) AND (Ocriplasmin OR Jetrea OR Microplasmin)
Appendix 7. WHO ICTRP search strategy
vitreomacular adhesion OR vitreomacular traction OR macular hole = Intervention AND Ocriplasmin OR Jetrea OR Microplasmin = Condition
Appendix 8. Data on study characteristics
| Mandatory items | Optional items | |
| Methods | ||
| Study design |
|
Exclusions after randomisation Losses to follow‐up Number randomised/analysed How were missing data handled? e.g. available case analysis, imputation methods Reported power calculation (Y/N), if yes, sample size and power Unusual study design/issues |
| Eyes or Unit of randomisation/unit of analysis |
|
|
| Participants | ||
| Country | Setting Ethnic group Equivalence of baseline characteristics (Y/N) | |
| Total number of participants | This information should be collected for total study population recruited into the study. If these data are only reported for the people who were followed up only, please indicate. | |
| Number (%) of men and women | ||
| Mean age and age range | ||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Interventions | ||
| Intervention (n = ) Comparator (n = ) See MECIR 65 and 70 |
|
|
| Outcomes | ||
| Primary and secondary outcomes as defined in study reports See MECIR R70 | List outcomes Adverse events reported (Y/N) Length of follow‐up and intervals at which outcomes assessed | Planned/actual length of follow‐up |
| Notes | ||
| Date conducted | Specify dates of recruitment of participants mm/yr to mm/yr | Full study name:(if applicable) Reported subgroup analyses (Y/N) Were trial investigators contacted? |
| Sources of funding | ||
| Declaration of interest See MECIR 69 |
||
MECIR: Methodological expectations for Cochrane Intervention Reviews; mm: month; n: number of participants; RCT: randomised controlled trial; yr: year.
Appendix 9. Glossary of abbreviations
BCVA: best corrected visual acuity. BEAVRS: British and Eire vitreoretinal surgeons. ERM: epiretinal membrane. ETDRS: Early Treatment Diabetic Retinopathy Study. GRADE: Grading of Recommendations, Assessment, Development and Evaluation working group. ILM: internal limiting membrane. logMAR: logarithm of the minimum angle of resolution. MH: macular hole. NEI‐VFQ 25: National Eye Institute Visual Function Questionnaire ‐ 25. NICE: National Institute for Health and Care Excellence. OCT: optical coherence tomography. PVD: posterior vitreous detachment. RCT: randomised controlled trial. sVMA: symptomatic vitreomacular adhesion. VFQ: Visual Function Questionnaire. VMA: vitreomacular adhesion. VMT: vitreomacular traction. WHO: World Health Organization.
Data and analyses
Comparison 1. Ocriplasmin versus sham injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete release of vitreous adhesion 28 days after treatment | 4 | 859 | Risk Ratio (M‐H, Random, 95% CI) | 3.46 [2.00, 6.00] |
| 2 Closure of macular hole 28 days after treatment | 3 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [1.50, 5.51] |
| 3 Complete posterior vitreous detachment 28 days after treatment | 3 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [1.39, 6.24] |
| 4 > 3‐line improvement in BCVA 6 months after treatment | 3 | 674 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [1.07, 3.53] |
| 5 Requirement for pars plana vitrectomy at month 6 | 3 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.50, 0.91] |
| 6 Any ocular adverse event | 4 | 909 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.09, 1.37] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
MIVI‐IIT 2010.
| Methods |
Study design: RCT, single treated eye. Number randomised: 60 total; 48 microplasmin; 12 sham injection. Exclusions after randomisation: none. Number analysed: at 28 days and 6 months; 60 total; 48 microplasmin; 12 sham injection. Unit of analysis: eyes. Losses to follow‐up: 0 participants total. How was missing data handled? no missing data. Power calculation: not documented. |
|
| Participants |
Country: Belgium. Mean age: 70.0 years overall; 69.9 years for ocriplasmin group; 70.0 years for sham injection group. Gender: 33/60 (55%) women, 27/60 (45%) men total; 27/48 (56%) women, 21/48 (44%) men in microplasmin group; 6/12 (50%) women, 6/12 (50%) men in sham injection group. Inclusion criteria: aged > 18 years; partial PVD on ultrasound examination; OCT evidence of at least a partial attachment in the foveal area, resulting in a macular thickness of ≥ 250 μm; BCVA ≤ 20/40 in study eye; BCVA ≥ 20/400 in fellow eye. Exclusion criteria: active PDR; high myopia (axial length > 26 mm); previous vitrectomy or uncontrolled glaucoma; previous intravitreal injections in the past 3 months in study eye; intraocular surgery or laser photocoagulation in the past 3 months in study eye; rhegmatogenous retinal detachment in either eye. Equivalence of baseline characteristics: no; more participants in microplasmin group had tractional diabetic macular oedema compared with sham injection group. |
|
| Interventions |
Intervention 1: single intravitreal injection microplasmin 125 µg. Intervention 2: single intravitreal injection microplasmin 75 µg. Intervention 3: single intravitreal injection microplasmin 175 µg. Intervention 4: intravitreal injection of microplasmin 125 µg at baseline, followed by a further microplasmin 125 µg intravitreal injection at 28 days if VMA was still present, followed by a further microplasmin 125 µg intravitreal injection at 56 days after baseline if VMA was still present. Comparator: sham injection (conjunctiva touched with a blunt needle by a non‐masked investigator and no injection given). Length of follow‐up: planned 180 days, actual 180 days. As the recommended dose of ocriplasmin is 125 µg, and this is the subject of this review, only data from the first and fourth intervention arms were analysed. |
|
| Outcomes |
Primary outcome, as defined in study reports: "the primary outcome of this study was total PVD induction at Day 14, as assessed by a central reading centre." Secondary outcomes, as defined in study reports: total PVD at other time points assessed by the central reading centre and investigators; resolution of index condition (VMA or MH); resolution of VMA; progression of PVD; need for vitrectomy; resolution of macular oedema; change in BCVA; BCVA 5‐, 10‐ and 15‐letter improvement. Adverse events reported: yes. Intervals at which outcomes assessed: 3, 7, 14, 28, 90 and 180 days. |
|
| Notes |
Funding sources: study sponsored by ThromboGenics NV. Study period: 2 years; 2007‐2009. Reported subgroup analyses: yes. Full results of study were presented at EURetina 2009, Nice, France. NCT00435539. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation for the MIVI‐IIT RCT not described. Quote: "Four cohorts of 15 patients were randomised as 4:1 to treatment or sham injection, resulting in 12 patients receiving microplasmin and 3 patients receiving the sham injection in each cohort." p. 1123. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information documented to assess allocation concealment. Quote: "Four cohorts of 15 patients were randomised as 4:1 to treatment or sham injection, resulting in 12 patients receiving microplasmin and 3 patients receiving the sham injection in each cohort." p. 1123. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Sham injection was performed, rather than actual placebo injection. Quote: "In the patients receiving a sham injection, microplasmin was prepared in the same manner, but instead of an intraocular injection, the conjunctiva was touched with a blunt needle by a nonmasked investigator and no injection was given." p. 1123. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All patient examinations before drug allocation and in the 6‐month follow‐up period after the last injection were performed by masked investigators and study personnel." p. 1123. "Posterior vitreous detachment status and macular thickness were assessed by the investigator as well as by a central reading center (CRC), located in Munich, Germany." p. 1124. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | All outcomes defined in trial registry were reported. |
| Other bias | Low risk | |
OASIS 2016.
| Methods |
Study design: RCT, single treated eye. Number randomised: 220 total; 146 ocriplasmin; 74 sham. Exclusions after randomisation: 50 participants subsequently deemed ineligible by central reading centre. Number analysed: at 28 days: 168 total; 111 ocriplasmin; 59 sham. Unit of analysis: eyes. Losses to follow‐up: 2 participants total; 1 ocriplasmin group (1 lost to follow‐up); 1 sham group (1 lost to follow‐up). How was missing data handled? other than VMA release at 28 days, no data published regarding cohort who met central reading centre eligibility criteria. Power calculation: 210 participants for at least 90% power at 2‐sided alpha of 0.05 to assume a primary endpoint of 37% in ocriplasmin group and a 14% rate in placebo group. |
|
| Participants |
Country: USA. Mean age: 69.1 years overall; 69.4 years for ocriplasmin group; 68.5 years for sham group. Gender: 147/218 (67.4%) women, 71/218 (32.6%) men total; 102/145 (70.3%) women, 43/145 (29.7%) men in ocriplasmin group; 45/73 (61.6%) women, 28/73 (38.4%) men in sham group. Inclusion criteria: aged > 18 years; presence of VMA; BCVA ≤ 20/32 in study eye; BCVA ≥ 20/800 in non‐study eye. Exclusion criteria: history or current evidence of proliferative retinopathy, exudative AMD or retinal vein occlusion in the study eye; people with any vitreous haemorrhage or any other vitreous opacification which precludes either visualisation of the posterior pole by visual inspection OR adequate assessment of the macula by OCT; MH > 400 µm in diameter in the study eye; presence of epiretinal membrane; aphakia in study eye; high myopia (> ‐8 dioptres in study eye); history of rhegmatogenous retinal detachment in either eye; prior vitrectomy in study eye; previous participation in this trial or prior administration of ocriplasmin in study eye. Equivalence of baseline characteristics: yes. |
|
| Interventions |
Intervention: single intravitreal injection of ocriplasmin 125 µg in 0.10 mL volume. Comparator: sham (the same syringe hub was pressed against the conjunctiva to simulate an injection). Length of follow‐up: planned 24 months, actual 24 months. Data of central reading centre approved study participants only reported at 28 days. |
|
| Outcomes |
Primary outcome, as defined in study reports: "proportion of subjects with pharmacological vitreomacular adhesion (VMA)/vitreomacular traction (VMT) resolution at day 28. Pharmacological VMA resolution without anatomical defect, based on spectral domain optical coherence tomography and determined by the masked central reading center (CRC), with post‐resolution vitrectomy considered as a failure." Secondary outcomes, as defined in study reports: "proportion of subjects with a ≥2 line improvement in best‐corrected visual acuity (BCVA) from baseline at month 24, irrespective of vitrectomy." Adverse events reported: yes. Intervals at which outcomes assessed: 7 and 28 days; 3, 6, 9, 12, 15, 18, 21 and 24 months. |
|
| Notes |
Funding sources: study sponsored by ThromboGenics NV. Study period: 3 years; 2011‐2014. Reported subgroup analyses: yes. Additional information: large proportion of participants were deemed eligible, recruited and treated by investigators. Retrospective central reading centre review found 50 participants ineligible for following reasons (MH > 400 μm, presence of epiretinal membrane or no sVMA at baseline). Our analysis only included data reported for correctly eligible cohort of participants. NCT01429441. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of random sequence generation for the MIVI‐IIT RCT described Quote: "Randomization was stratified on the basis of the presence or absence of FTMH at baseline and was centralized through an interactive voice response system." p. 2233. |
| Allocation concealment (selection bias) | Low risk | Method of allocation concealment for MIVI‐IIT RCT described. Quote: "Randomization was stratified on the basis of the presence or absence of FTMH at baseline and was centralized through an interactive voice response system." p. 2233. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Performance bias explained. Quote: "The trial was conducted in a double‐masked manner. To maintain the masking of the investigator, an unmasked injecting physician was assigned to perform the injection and access the interactive voice response system to receive the assigned treatment. The unmasked personnel did not perform or participate in any other trial‐related procedures or assessments." p. 2233. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Detection bias appropriately explained. Quote: "The trial was conducted in a double‐masked manner. To maintain the masking of the investigator, an unmasked injecting physician was assigned to perform the injection and access the interactive voice response system to receive the assigned treatment. The unmasked personnel did not perform or participate in any other trial‐related procedures or assessments." p. 2233. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Large proportion of participants deemed eligible, recruited and treated by investigators. Retrospective central reading centre review found 50 participants ineligible for following reasons (MH > 400 μm, presence of epiretinal membrane or no sVMA). Outcome data for correct eligible cohort of participants only given for primary outcome. No secondary outcome data described. |
| Selective reporting (reporting bias) | Low risk | All outcomes defined in trial registry were reported. |
| Other bias | Low risk | No other source of bias. |
TG‐MV‐006 2012.
| Methods |
Study design: RCT, single treated eye. Number randomised: 326 total; 219 ocriplasmin; 107 placebo. Exclusions after randomisation: 0. Number analysed: at 28 days: 326 total; 219 ocriplasmin; 107 placebo. At 180 days: 298 total; 200 ocriplasmin; 98 placebo. Unit of analysis: eyes. Losses to follow‐up: 28 participants total; 19 ocriplasmin group (2 adverse event, 8 withdrawal by participants, 6 lost to follow‐up, 3 death); 9 placebo group (2 adverse event, 4 withdrawal by participants, 3 lost to follow‐up). How was missing data handled? missing data not reported in study publications. Power calculation: 320 participants for > 90% power at 2‐sided alpha of 0.05 to assume a primary endpoint of 27.5% in ocriplasmin group and 10.0% in placebo group. |
|
| Participants |
Country: USA. Mean age: 71.4 years overall; 71.5 years for ocriplasmin group; 71.1 years for placebo group. Gender: 207/326 (63.5%) women, 119/326 (36.5%) men total; 148/219 (67.6%) women, 71/219 (32.4%) men in ocriplasmin group; 59/107 (55.1%) women, 52/107 (48.6%) men in placebo group. Inclusion criteria: aged > 18 years; focal VMA (vitreous adhesion to macula within 6‐mm central retinal field surrounded by elevation of posterior vitreous cortex, as seen on OCT that in the opinion of investigator was related to decreased visual function (e.g. metamorphopsia, decreased visual acuity or other visual complaint); BCVA ≤ 20/25 in study eye; BCVA ≥ 20/800 in non‐study eye. Exclusion criteria: any evidence of proliferative retinopathy (including PDR or other ischaemic retinopathies involving vitreoretinal vascular proliferation) or exudative AMD or retinal vein occlusion in study eye; people with any vitreous haemorrhage or any other vitreous opacification which precludes either: visualisation of posterior pole by visual inspection OR adequate assessment of macula by either OCT or fluorescein angiogram (or both) in study eye; MH > 400 µm in diameter in study eye; aphakia in study eye; high myopia (> ‐8 dioptres); uncontrolled glaucoma; lenticular or zonular instability; history of retinal detachment in either eye; prior vitrectomy or prior laser photocoagulation of macula; treatment with ocular surgery, intravitreal injection or retinal laser photocoagulation in the previous 3 months. Equivalence of baseline characteristics: no; pseudophakia more common in ocriplasmin group than in placebo group; more women in ocriplasmin group than in placebo group. |
|
| Interventions |
Intervention: single intravitreal injection of ocriplasmin 125 µg in 0.10 mL volume. Comparator: single intravitreal injection of 0.10 mL placebo with identical drug vehicle diluted with saline. Length of follow‐up: planned 180 days, actual 180 days. |
|
| Outcomes |
Primary outcome, as defined in study reports: "the primary end point was the proportion of subjects with nonsurgical resolution of vitreomacular adhesion at day 28 post‐injection, as determined by masked OCT evaluation obtained from the central reading centre." Secondary outcomes, as defined in study reports: proportion of participants with total PVD at day 28, as determined by B‐scan ultrasound; need for vitrectomy; closure of an MH; gain ≥ 3‐lines BCVA without vitrectomy; change from baseline in BCVA and VFQ‐25 score at 6 months. Adverse events reported: yes. Intervals at which outcomes assessed: 7, 14, 28, 90 and 180 days. |
|
| Notes |
Funding sources: study sponsored by ThromboGenics NV. Study period: 2 years; 2008‐2009. Reported subgroup analyses: yes. NCT00781859. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Supplementary material: "subjects will be randomised centralized through a telephone‐based Interactive Voice Response System (IVRS) to either microplasmin intravitreal injection or placebo in a 3:1 allocation ratio. Blocks of treatment will be assigned to sites in a manner expected to minimize the potential for imbalance in the desired randomization ratio." Protocol p. 17. |
| Allocation concealment (selection bias) | Low risk | Supplementary material: "subjects will be randomised centralized through a telephone‐based Interactive Voice Response System (IVRS) to either microplasmin intravitreal injection or placebo in a 3:1 allocation ratio. Blocks of treatment will be assigned to sites in a manner expected to minimize the potential for imbalance in the desired randomization ratio." Protocol p. 17. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients randomly assigned to the ocriplasmin group received an intravitreal injection of ocriplasmin (125 μg in a 0.10‐ml volume) drawn from a vial containing ocriplasmin into which 0.75 ml of commercial saline had been injected (1875 μg of ocriplasmin in a 0.75‐ml drug vehicle). Patients randomly assigned to placebo received an intravitreal injection of 0.10 ml of the identical drug vehicle diluted with saline, the method used being the same as that used to prepare ocriplasmin." p. 608. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Trained readers at a central reading center (Duke University OCT Reading Center, Durham, NC) who were unaware of the group assignments evaluated the OCT images. All ultrasonographic studies were standardized and performed by certified technicians who underwent special training for the study. Staging of posterior vitreous detachment was based on dynamic ultrasonographic evaluation and performed by an investigator who was unaware of the group assignments." p. 608. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description found in article, but 28 participants reported as not completing study on ClinicalTrials.gov. The corresponding author of Stalmans and colleagues (2012) was contacted to query this. ThromboGenics NV responded by confirming use of last observation carried forward (LOCF) approach to input missing data for visits postdiscontinuation. Their explanation was: "use of LOCF was appropriate when the outcome is not expected to change after discontinuation and is a conservative method when the outcome is expected to improve spontaneously over time. The primary endpoint, pharmacological VMA resolution in particular, is an outcome of that nature." As these losses to follow‐up were not reported in original paper, risk of attrition bias was deemed unclear. |
| Selective reporting (reporting bias) | Low risk | All defined outcomes in methods were reported. |
| Other bias | Unclear risk | Baseline imbalance between study groups as pseudophakia was more common in ocriplasmin group than in placebo group and there were more women in ocriplasmin group than in placebo group. |
TG‐MV‐007 2012.
| Methods |
Study design: RCT, single treated eye. Number randomised: 326 total; 245 ocriplasmin; 81 placebo. Exclusions after randomisation: none. Number analysed: at 28 days: 326 total; 245 ocriplasmin; 81 placebo. At 180 days: 309 total; 235 ocriplasmin; 74 placebo. Unit of analysis: eyes. Losses to follow‐up: 17 participants total; 10 ocriplasmin group (5 withdrawal by participant, 2 lost to follow‐up, 2 adverse event, 1 death); 7 placebo group (1 physician decision, 4 withdrawal by participant, 2 lost to follow‐up). How was missing data handled? missing data not reported in study publications. Power calculation: 320 participants for > 90% power at 2‐sided alpha of 0.05 to assume a primary endpoint of 27.5% in ocriplasmin group and 10.0% rate in placebo group. |
|
| Participants |
Countries: Belgium, Czech Republic, Germany, Poland, Spain, UK, USA. Mean age: 72.0 years overall; 72.6 years for ocriplasmin group; 70.2 years for placebo group. Gender: 222/326 (68.1%) women, 104/326 (31.9%) men total; 166/245 (67.8%) women, 79/245 (32.2%) men in ocriplasmin group; 56/81 (69.1%) women, 25/81 (30.9%) men in placebo group. Inclusion criteria: aged > 18 years; focal VMA (vitreous adhesion to macula within 6‐mm central retinal field surrounded by elevation of posterior vitreous cortex, as seen on OCT that in the opinion of investigator was related to decreased visual function (e.g. metamorphopsia, decreased visual acuity or other visual complaint); BCVA ≤ 20/25 in study eye; BCVA ≥ 20/800 in non‐study eye. Exclusion criteria: any evidence of proliferative retinopathy (including PDR or other ischaemic retinopathies involving vitreoretinal vascular proliferation) or exudative AMD or retinal vein occlusion in study eye; people with any vitreous haemorrhage or any other vitreous opacification which precludes either: visualisation of posterior pole by visual inspection OR adequate assessment of macula by either OCT or fluorescein angiogram (or both) in study eye; MH > 400 µm in diameter in study eye; aphakia in study eye; high myopia (> ‐8 dioptres); uncontrolled glaucoma; lenticular or zonular instability; history of retinal detachment in either eye; prior vitrectomy or prior laser photocoagulation of macula; treatment with ocular surgery, intravitreal injection or retinal laser photocoagulation in the previous 3 months. Equivalence of baseline characteristics: no; pseudophakia more common in ocriplasmin group than in placebo group; more women in ocriplasmin group than in placebo group. |
|
| Interventions |
Intervention: single intravitreal injection of ocriplasmin 125 µg in 0.10 mL volume. Comparator: single intravitreal injection of 0.10 mL placebo with identical drug vehicle diluted with saline. Length of follow‐up: planned 180 days, actual 180 days. |
|
| Outcomes |
Primary outcome, as defined in study reports: "the primary end point was the proportion of subjects with nonsurgical resolution of VMA at day 28 post‐injection, as determined by masked OCT evaluation obtained from the central reading centre." Secondary outcomes, as defined in study reports: proportion of participants with total PVD at day 28, as determined by B‐scan ultrasound; need for vitrectomy; closure of an MH; gain ≥ 3‐lines BCVA without vitrectomy; change from baseline in BCVA and VFQ‐25 score at 6 months. Adverse events reported: yes. Intervals at which outcomes assessed: 7, 14, 28, 90 and 180 days. |
|
| Notes |
Funding sources: study sponsored by ThromboGenics NV. Study period: 2 years; 2008‐2010. Reported subgroup analyses: yes. NCT00798317. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Supplementary material: "subjects will be randomised centralized through a telephone‐based Interactive Voice Response System (IVRS) to either microplasmin intravitreal injection or placebo in a 3:1 allocation ratio. Blocks of treatment will be assigned to sites in a manner expected to minimize the potential for imbalance in the desired randomization ratio." Protocol p. 17. |
| Allocation concealment (selection bias) | Low risk | Supplementary material: "subjects will be randomised centralized through a telephone‐based Interactive Voice Response System (IVRS) to either microplasmin intravitreal injection or placebo in a 3:1 allocation ratio. Blocks of treatment will be assigned to sites in a manner expected to minimize the potential for imbalance in the desired randomization ratio." Protocol p. 17. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients randomly assigned to the ocriplasmin group received an intravitreal injection of ocri‐ plasmin (125μg in a 0.10‐ml volume) drawn from a vial containing ocriplasmin into which 0.75ml of commercial saline had been injected (1875μg of ocriplasmin in a 0.75‐ml drug vehicle). Patients randomly assigned to placebo received an intravitreal injection of 0.10 ml of the identical drug vehicle diluted with saline, the method used being the same as that used to prepare ocriplasmin". p. 608. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Trained readers at a central reading center (Duke University OCT Reading Center, Durham, NC) who were unaware of the group assignments evaluated the OCT images. All ultrasonographic studies were standardized and performed by certified technicians who underwent special training for the study. Staging of posterior vitreous detachment was based on dynamic ultrasonographic evaluation and performed by an investigator who was unaware of the group assignments." p. 608. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description found in article, but 17 participants reported as not completing study on ClinicalTrials.gov. The corresponding author of Stalmans and colleagues (2012) was contacted to query this. ThromboGenics NV responded by confirming use of last observation carried forward (LOCF) approach to input missing data for visits postdiscontinuation. Their explanation was: "use of LOCF was appropriate when the outcome is not expected to change after discontinuation and is a conservative method when the outcome is expected to improve spontaneously over time. The primary endpoint, pharmacological VMA resolution in particular, is an outcome of that nature." As these losses to follow‐up were not reported in original paper, risk of attrition bias was deemed unclear. |
| Selective reporting (reporting bias) | Low risk | All defined outcomes in methods were reported. |
| Other bias | Unclear risk | Baseline imbalance between study groups as pseudophakia was more common in ocriplasmin group than in placebo group and there were more women in ocriplasmin group than in placebo group. |
AMD: age‐related macular degeneration; BCVA: best corrected visual acuity; FTMH: full‐thickness macular hole; MH: macular hole; OCT: optical coherence tomography; PDR: proliferative diabetic retinopathy; PVD: posterior vitreous detachment; RCT: randomised controlled trial; sVMA: symptomatic vitreomacular adhesion; VFQ‐25: Visual Function Questionnaire 25; VMA: vitreomacular adhesion.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Benz 2010 | Indication for ocriplasmin was not symptomatic vitreomacular adhesion. It was investigating whether 125 µg microplasmin would induce vitreous release in people scheduled for PPV. |
| De Smet 2009 | Investigated safety and efficacy of 4 different doses of intravitreal microplasmin prior to preplanned PPV. Subsequent PPV occurred either 1‐2 hours, 24 hours or 7 days following ocriplasmin, meaning the participant population and outcome measures were not eligible for inclusion in our review. |
| Dugel 2015 | Post hoc analysis of data from studies we already extracted data from (TG‐MV‐006 and TG‐MV‐007). |
| Elbendary 2011 | Autologous plasmin injected into participants with diabetic macular oedema associated with vitreomacular traction. |
| Jackson 2017 | Incorrect study design; post hoc analysis. |
| Lanzetta 2014a | Postmarket surveillance study, not an RCT, therefore not eligible for inclusion. |
| Lanzetta 2014b | Post‐hoc analysis of data, not an RCT, therefore excluded. |
| Lescrauwaet 2016 | Not an RCT. |
| Novack 2015 | Eligible participants for this study required exudative age‐related macular degeneration, which did not meet inclusion criteria for our review. |
PPV: pars plana vitrectomy; RCT: randomised controlled trial.
Differences between protocol and review
Due to the reporting of BCVA in the included studies, we changed our secondary outcome measure from "mean change in BCVA" to "proportion gaining 3‐line or greater improvement in VA, measured using the ETDRS scale".
We added a secondary outcome measure, the requirement of PPV. This gives a good measure of how successful the intervention of ocriplasmin has been (i.e. conventional treatment for sVMA has been PPV, and indeed this remains the treatment modality of choice who fail ocriplasmin therapy).
We added information regarding "other bias" that could not be accurately categorised under the other categories of bias.
Contributions of authors
JN: wrote the first draft of the protocol and review and reviewed the electronic search results. VK: reviewed the electronic search results, carried out the statistical analysis and critically appraised the review. EP: critically appraised the review. TJ: reviewed the electronic search results, and critically appraised all drafts of the protocol and review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
JN: financial support to attend conferences (COPHy 2014 ‐ ThromboGenics; AAO 2016, Retinal World Congress 2017 ‐ VisionCare). VK: financial support to attend conferences (ARVO 2017 ‐ Bayer). EP: no disclosures. TJ: received an honorarium in 2016 to attend the EVER conference in Nice: ThromboGenics; advisory boards: Alcon, Bausch & Lomb, DORC; research grant support (employer): Novartis.
New
References
References to studies included in this review
MIVI‐IIT 2010 {published data only}
- Stalmans P, Delaey C, Smet MD, Dijkman E, Pakola S. Intravitreal injection of microplasmin for treatment of vitreomacular adhesion: results of a prospective, randomized, sham‐controlled phase II trial (the MIVI‐IIT trial). Retina 2010;30(7):1122‐7. [DOI] [PubMed] [Google Scholar]
OASIS 2016 {published data only}
- Dugel PU, Tolentino M, Feiner L, Kozma P, Leroy A. Results of the 2‐year ocriplasmin for treatment for symptomatic vitreomacular adhesion including macular hole (OASIS) randomized trial. Ophthalmology 2016;123(10):2232‐47. [DOI] [PubMed] [Google Scholar]
TG‐MV‐006 2012 {published data only}
- Haller JA, Stalmans P, Benz MS, Gandorfer A, Pakola SJ, Girach A, et al. Efficacy of intravitreal ocriplasmin for treatment of vitreomacular adhesion: subgroup analyses from two randomized trials. Ophthalmology 2015;122(1):117‐22. [DOI] [PubMed] [Google Scholar]
- Stalmans P, Benz MS, Gandorfer A, Kampik A, Girach A, Pakola S, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. New England Journal of Medicine 2012;367(7):606‐15. [DOI] [PubMed] [Google Scholar]
- Varma R, Haller JA, Kaiser PK. Improvement in patient‐reported visual function after ocriplasmin for vitreomacular adhesion: results of the microplasmin for intravitreous injection‐traction release without surgical treatment (MIVI‐TRUST) trials. JAMA Ophthalmology 2015;133(9):997‐1004. [DOI] [PubMed] [Google Scholar]
TG‐MV‐007 2012 {published data only}
- Haller JA, Stalmans P, Benz MS, Gandorfer A, Pakola SJ, Girach A, et al. Efficacy of intravitreal ocriplasmin for treatment of vitreomacular adhesion: subgroup analyses from two randomized trials. Ophthalmology 2015;122(1):117‐22. [DOI] [PubMed] [Google Scholar]
- Stalmans P, Benz MS, Gandorfer A, Kampik A, Girach A, Pakola S, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. New England Journal of Medicine 2012;367(7):606‐15. [DOI] [PubMed] [Google Scholar]
- Varma R, Haller JA, Kaiser PK. Improvement in patient‐reported visual function after ocriplasmin for vitreomacular adhesion: results of the microplasmin for intravitreous injecion‐traction release without surgical treatment (MIVI‐TRUST) trials. JAMA Ophthalmology 2015;133(9):997‐1004. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Benz 2010 {published data only}
- Benz, MS, Packo KH, Gonzalez V, Pakola S, Bezner D, Haller JA, et al. A placebo‐controlled trial of microplasmin intravitreous injection to facilitate posterior vitreous detachment before vitrectomy. Ophthalmology 2010;117(4):791‐7. [DOI] [PubMed] [Google Scholar]
De Smet 2009 {published data only}
- Smet MD, Gandorfer A, Stalmans P, Veckeneer M, Feron E, Pakola S, et al. Microplasmin intravitreal administration in patients with vitreomacular traction scheduled for vitrectomy: the MIVI I trial. Ophthalmology 2009;116(7):1349‐55. [DOI] [PubMed] [Google Scholar]
Dugel 2015 {published data only}
- Dugel PU, Regillo C, Eliott D. Characterization of anatomic and visual function outcomes in patients with full‐thickness macular hole in ocriplasmin phase 3 trials. American Journal of Ophthalmology 2015;160(1):94‐9. [DOI] [PubMed] [Google Scholar]
Elbendary 2011 {published data only}
- Elbendary AM, Elwan MM, Azzam HA, Eldeeb DR. Predictability of vitreous detachment following intravitreal plasmin injection in diabetic macular edema associated with vitreomacular traction. Current Eye Research 2011;36(6):534‐9. [DOI] [PubMed] [Google Scholar]
Jackson 2017 {published data only}
- Jackson TL, Verstraeten T, Duchateau L, Lescrauwaet B. Visual function response to ocriplasmin for the treatment of vitreomacular traction and macular hole. Acta Ophthalmologica 2017 Jan 30 [Epub ahead of print]. [DOI: 10.1111/aos.13369] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lanzetta 2014a {published data only}
- Lanzetta P. Ocriplasmin safety overview from clinical trials and postmarketing data. Ophthalmologica 2014;232:65. [Google Scholar]
Lanzetta 2014b {published data only}
- Lanzetta P. Anatomic and functional responses in clinically relevant subgroups of the MIVI‐TRUST clinical trials after ocriplasmin treatment. Ophthalmologica 2014;232:66. [Google Scholar]
Lescrauwaet 2016 {published data only}
- Lescrauwaet B, Duchateau L, Verstraeten T, Jackson TL. Long‐term visual functioning improvement is associated with resolution of vitreomacular adhesion in subjects with vitreomacular traction, including macular hole treated with intravitreal ocriplasmin: results from the oasis study. International Society for Pharmacoeconomics and Outcomes Research 19th Annual European Congress; 2016 Oct 29 ‐ Nov 2; Vienna, Austria.
Novack 2015 {published data only}
- Novack RL, Staurenghi G, Girach A, Narendran N, Tolentino M. Safety of intravitreal ocriplasmin for focal vitreomacular adhesion in patients with exudative age‐related macular degeneration. Ophthalmology 2015;122(4):796‐802. [DOI] [PubMed] [Google Scholar]
Additional references
Almeida 2015
- Almeida DR, Chin EK, Rahim K, Folk JC, Russell SR. Factors associated with spontaneous release of vitreomacular traction. Retina 2015;35(3):492‐7. [DOI] [PubMed] [Google Scholar]
Chan 1995
- Chan CK, Wessels IF, Friedrichsen EJ. Treatment of idiopathic macular holes by induced posterior vitreous detachment. Ophthalmology 1995;102(5):757‐67. [DOI] [PubMed] [Google Scholar]
Chauhan 2000
- Chauhan DS, Antcliff RJ, Rai PA, Williamson TH, Marshall J. Papillofoveal traction in macular hole formation: the role of optical coherence tomography. Archives of Ophthalmology 2000;118(1):32‐8. [DOI] [PubMed] [Google Scholar]
Foos 1982
- Foos RY, Wheeler NC. Vitreoretinal juncture. Synchysis senilis and posterior vitreous detachment. Ophthalmology 1982;89(12):1502‐12. [DOI] [PubMed] [Google Scholar]
Gandorfer 2008
- Gandorfer A. Enzymatic vitreous disruption. Eye 2008;22(10):1273‐7. [DOI] [PubMed] [Google Scholar]
Gass 1988
- Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Archives of Ophthalmology 1988;106(5):62‐39. [DOI] [PubMed] [Google Scholar]
Gaudric 1999
- Gaudric A, Haouchine B, Massin P, Paques M, Blain P, Erqinay A. Macular hole formation: new data provided by optical coherence tomography. Archives of Ophthalmology 1999;117(6):744‐51. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed prior to 19 April 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Hahn 2015
- Hahn P, Chung MM, Flynn HW Jr, Huang SS, Kim JE, Mahmoud TH, et al. Safety profile of ocriplasmin for symptomatic vitreomacular adhesion: a comprehensive analysis of premarketing and postmarketing experiences. Retina 2015;35(6):1128‐34. [DOI] [PubMed] [Google Scholar]
Haller 2015
- Haller JA, Stalmans P, Benz MS, Gandorfer A, Pakola SJ, Girach A, et al. Efficacy of intravitreal ocriplasmin for treatment of vitreomacular adhesion: subgroup analyses from two randomized trials. Ophthalmology 2015;122(1):117‐22. [DOI] [PubMed] [Google Scholar]
Haynes 2017
- Haynes RJ, Yorston D, Laidlaw DA, Keller J, Steel DH. Real world outcomes of ocriplasmin use by members of the British and Eire Association of Vitreoretinal Surgeons. Eye 2017;31(1):107‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hikichi 1995
- Hikichi T, Yoshida A, Akiba J, Trempe CL. Natural outcomes of stage 1, 2, 3, and 4 idiopathic macular holes. British Journal of Ophthalmology 1995;79(6):517‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ito 2003
- Ito Y, Terasaki H, Suzuki T, Kojima T, Mori M, Ishikawa K, et al. Mapping posterior vitreous detachment by optical coherence tomography in eyes with idiopathic macular hole. American Journal of Ophthalmology 2003;135(3):351‐5. [DOI] [PubMed] [Google Scholar]
Jackson 2013a
- Jackson TL, Nicod E, Simpson A, Angelis A, Grimaccia F, Kanavos P. Symptomatic vitreomacular adhesion. Retina 2013;33(8):1503‐11. [DOI] [PubMed] [Google Scholar]
Jackson 2013b
- Jackson TL, Nicod E, Angelis A, Grimaccia F, Prevost AT, Simpson AR, et al. Vitreous attachment in age‐related macular degeneration, diabetic macular edema, and retinal vein occlusion: a systematic review and metaanalysis. Retina 2013;33(6):1099‐108. [DOI] [PubMed] [Google Scholar]
Jackson 2013c
- Jackson TL, Donachie PH, Sparrow JM, Johnston RL. United Kingdom National Ophthalmology Database Study of Vitreoretinal Surgery: report 1; case mix, complications, and cataract. Eye 2013;27(5):644‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 2016
- Jackson TL, Regillo CD, Girach A, Dugel PU, MIVI‐TRUST Study Group. Baseline predictors of vitreomacular adhesion/traction resolution following an intravitreal injection of ocriplasmin. Ophthalmic Surgery, Lasers and Imaging Retina 2016;47(8):716‐23. [DOI] [PubMed] [Google Scholar]
Johnson 2010
- Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. American Journal of Ophthalmology 2010;149(3):371‐82. [DOI] [PubMed] [Google Scholar]
Khan 2016
- Khan MA, Haller JA. Ocriplasmin for treatment of vitreomacular traction: an update. Ophthalmology and Therapy 2016;5(2):147‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuppermann 2012
- Kuppermann BD. Ocriplasmin for pharmacologic vitreolysis. Retina 2012;32(Suppl 2):S225‐8. [DOI] [PubMed] [Google Scholar]
La Cour 2002
- Cour M, Friis J. Macular holes: classification, epidemiology, natural history and treatment. Acta Ophthalmologica Scandinavica 2002;80(6):579‐87. [DOI] [PubMed] [Google Scholar]
Margherio 1998
- Margherio AR, Margherio RR, Hartzer M, Trese MT, Williams GA, Ferrone PJ. Plasmin enzyme‐assisted vitrectomy in traumatic pediatric macular holes. Ophthalmology 1998;105(9):1617‐20. [DOI] [PubMed] [Google Scholar]
Melberg 1995
- Melberg NS, Williams DF, Balles MW, Jaffe GJ, Meredith TA, Sneed SR, et al. Vitrectomy for vitreomacular traction syndrome with macular detachment. Retina 1995;15(3):192‐7. [DOI] [PubMed] [Google Scholar]
Mori 2007
- Mori K, Saito S, Gehlbach PL, Yoneya S. Treatment of stage 2 macular hole by intravitreous injection of expansile gas and induction of posterior vitreous detachment. Ophthalmology 2007;114(1):127‐33. [DOI] [PubMed] [Google Scholar]
Neffendorf 2016
- Neffendorf JE, Lim LT, Gout II, El‐Amir A. Widespread macular neurosensory detachment after ocriplasmin intravitreal injection. Retinal Cases and Brief Reports 2016;10(4):354‐6. [DOI] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence. Ocriplasmin for treating vitreomacular traction. NICE technology appraisal guidance 297. www.nice.org.uk/guidance/ta297 (accessed 8 September 2017).
Nomura 2014
- Nomura Y, Takahashi H, Tan X, Fujimora S, Obata R, Yanagi Y. Effects of vitreomacular adhesion on ranibizumab treatment in Japanese patients with age‐related macular degeneration. Japanese Journal of Ophthalmology 2014;58(5):443‐7. [DOI] [PubMed] [Google Scholar]
Paul 2017
- Paul C, Heun C, Müller HH, Fauser S, Kaymak H, Kazerounian S, et al. Impact of vitreoretinal interface architecture on successful vitreomacular traction resolution in eyes scheduled for intravitreal ocriplasmin therapy. Retina 2017;37(7):1252‐60. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodrigues 2013
- Rodrigues IA, Stangos AN, McHugh DA, Jackson TL. Intravitreal injection of expansile perfluoropropane (c(3)f(8)) for the treatment of vitreomacular traction. American Journal of Ophthalmology 2013;155(2):270‐6. [DOI] [PubMed] [Google Scholar]
Sakuma 2006
- Sakuma T, Tanaka M, Inoue J, Mizota A, Souri M, Ichinose A. Use of autologous plasmin during vitrectomy for diabetic maculopathy. European Journal of Ophthalmology 2006;16(1):138‐40. [DOI] [PubMed] [Google Scholar]
Simpson 2012
- Simpson AR, Petrarca R, Jackson TL. Vitreomacular adhesion and neovascular age‐related macular degeneration. Survey of Ophthalmology 2012;57(6):498‐509. [DOI] [PubMed] [Google Scholar]
SmPC 2013
- JETREA 0.5 mg/0.2 ml concentrate for solution for injection. www.medicines.org.uk/emc/medicine/27585 (accessed 10 September 2015).
Sonmez 2008
- Sonmez K, Capone A Jr, Trese MT, Williams GA. Vitreomacular traction syndrome: impact of anatomical configuration on anatomical and visual outcomes. Retina 2008;28(9):1207‐14. [DOI] [PubMed] [Google Scholar]
Stalmans 2010
- Stalmans P, Delaey C, Smet MD, Dijkman E, Pakola S. Intravitreal injection of microplasmin for treatment of vitreomacular adhesion: results of a prospective, randomized, sham‐controlled phase II trial (the MIVI‐IIT trial). Retina 2010;30(7):1122‐7. [DOI] [PubMed] [Google Scholar]
Stalmans 2012
- Stalmans P, Benz MS, Gandorfer A, Kampik A, Girach A, Pakola S, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. New England Journal of Medicine 2012;367(7):606‐15. [DOI] [PubMed] [Google Scholar]
Steel 2013
- Steel DH, Lotery AJ. Idiopathic vitreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye 2013;27(Suppl 1):S1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanner 2001
- Tanner V, Chauhan DS, Jackson TL, Williamson TH. Optical coherence tomography of the vitreoretinal interface in macular hole formation. British Journal of Ophthalmology 2001;85(9):1092‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Terao 2014
- Terao R, Yuda K, Kure K, Inoue T, Ohtsu H, Yanagi Y. Effect of vitreomacular adhesion on antivascular endothelial growth factor therapy for macular edema secondary to branch retinal vein occlusion. Japanese Journal of Ophthalmology 2014;58(2):139‐45. [DOI] [PubMed] [Google Scholar]
Theodossiadis 2014
- Theodossiadis GP, Grigoropoulos VG, Theodoropoulou S, Datseris I, Theodossiadis PG. Spontaneous release of vitreomacular traction demonstrated by spectral‐domain optical coherence tomography. American Journal of Ophthalmology 2014;157(4):842‐51. [DOI] [PubMed] [Google Scholar]
Uchino 2001
- Uchino E, Uemura A, Ohba N. Initial stages of posterior vitreous detachment in healthy eyes of older persons evaluated by optical coherence tomography. Archives of Ophthalmology 2001;119(10):1475‐9. [DOI] [PubMed] [Google Scholar]
Varma 2015
- Varma R, Haller JA, Kaiser PK. Improvement in patient‐reported visual function after ocriplasmin for vitreomacular adhesion: results of the microplasmin for intravitreous injecion‐traction release without surgical treatment (MIVI‐TRUST) trials. JAMA Ophthalmology 2015;133(9):997‐1004. [DOI] [PubMed] [Google Scholar]
Waldstein 2014
- Waldstein SM, Ritter M, Simader C, Mayr‐Sponer U, Kundi M, Schmidt‐Erfurth U. Impact of vitreomacular adhesion on ranibizumab mono‐ and combination therapy for neovascular age‐related macular degeneration. American Journal of Ophthalmology 2014;158(2):328‐36. [DOI] [PubMed] [Google Scholar]
Weinard 2009
- Weinard F, Jung A, Becker R, Pavlovic S. Spontaneous resolution of vitreomacular traction syndrome. Ophthalmologe 2009;106(1):44‐6. [DOI] [PubMed] [Google Scholar]
Westheimer 1979
- Westheimer G. Scaling of visual acuity measurements. Archives of Ophthalmology 1979;97(2):327‐30. [DOI] [PubMed] [Google Scholar]
Williams 2001
- Williams JG, Trese MT, Williams GA, Hartzer MK. Autologous plasmin enzyme in the surgical management of diabetic retinopathy. Ophthalmology 2001;108(10):1902‐5. [DOI] [PubMed] [Google Scholar]
Yoon 2014
- Yoon D, Rusu I, Barbazetto I. Reduced effect of anti‐vascular endothelial growth factor agents on diabetics with vitreomacular interface abnormalities. International Ophthalmology 2014;34(4):817‐23. [DOI] [PubMed] [Google Scholar]
Zhang 2015
- Zhang Z, Dong F, Zhao C, Dai R, Yu W, Zheng L, et al. Natural course of vitreomacular traction syndrome observed by spectral‐domain optical coherence tomography. Canadian Journal of Ophthalmology 2015;50(2):172‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Neffendorf 2015
- Neffendorf JE, Pringle E, Jackson TL. Ocriplasmin for symptomatic vitreomacular adhesion. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD011874] [DOI] [PMC free article] [PubMed] [Google Scholar]


