Abstract
Communication requires the abilities to generate and interpret utterances and to infer the beliefs, desires, and goals of others (“Theory of Mind”; ToM). These two abilities have been shown to dissociate: individuals with aphasia retain the ability to think about others’ mental states; and individuals with autism are impaired in social reasoning, but their basic language processing is often intact. In line with this evidence from brain disorders, functional MRI (fMRI) studies have shown that linguistic and ToM abilities recruit distinct sets of brain regions. And yet, language is a social tool that allows us to share thoughts with one another. Thus, the language and ToM brain networks must share information despite being implemented in distinct neural circuits. Here, we investigated potential interactions between these networks during naturalistic cognition using functional correlations in fMRI. The networks were functionally defined in individual participants, in terms of preference for sentences over nonwords for language, and for belief inference over physical-event processing for ToM, with both a verbal and a nonverbal paradigm. Although, across experiments, interregion correlations within each network were higher than between-network correlations, we also observed above-baseline synchronization of blood oxygenation level-dependent signal fluctuations between the two networks during rest and story comprehension. This synchronization was functionally specific: neither network was synchronized with the executive control network (functionally defined in terms of preference for a harder over easier version of an executive task). Thus, coordination between the language and ToM networks appears to be an inherent and specific characteristic of their functional architecture.
NEW & NOTEWORTHY Humans differ from nonhuman primates in their abilities to communicate linguistically and to infer others’ mental states. Although linguistic and social abilities appear to be interlinked onto- and phylogenetically, they are dissociated in the adult human brain. Yet successful communication requires language and social reasoning to work in concert. Using functional MRI, we show that language regions are synchronized with social regions during rest and language comprehension, pointing to a possible mechanism for internetwork interaction.
Keywords: communication, fMRI, functional connectivity, language, Theory of Mind
INTRODUCTION
Language is the primary means for human interaction, and communicative success requires an ability to reason about a conversation partner’s beliefs, desires, and goals (e.g., Grice 1957, 1968, 1975; Sperber and Wilson 1986). This ability to make inferences about others’ mental states is often referred to as “mentalizing” or “Theory of Mind” (ToM). Two kinds of evidence suggest that in a mature human brain the cognitive and neural mechanisms that support ToM reasoning are dissociated from basic language processing mechanisms. First, patients with aphasia experience significant difficulty understanding and/or producing language but appear to be largely unimpaired in their ToM reasoning (e.g., Apperly et al. 2006; Dronkers et al. 1998; Varley and Siegal 2000; Varley et al. 2001; Willems et al. 2011). Conversely, individuals with autism spectrum disorders suffer from ToM—or more generalized social—deficits (e.g., Baron-Cohen et al. 1985; Happé 1993) yet often have their core linguistic processing abilities intact (e.g., Åsberg 2010; Diehl et al. 2006; Frith and Happé 1994; Janke and Perovic 2015; Lord and Paul 1997; Tager-Flusberg 2006; Tager-Flusberg et al. 2005; Terzi et al. 2016; Wilkinson 1998). And second, neuroimaging studies with healthy adults have revealed that largely distinct sets of brain regions support ToM versus language processing. Specifically, ToM reasoning engages bilateral regions at the junction of temporal and parietal lobes and a set of regions along the cortical midline (e.g., Ciaramidaro et al. 2007; Fletcher et al. 1995; Gallagher et al. 2000; Gobbini et al. 2007; Jacoby et al. 2016; Ruby and Decety 2003; Saxe and Kanwisher 2003; Vogeley et al. 2001; see, e.g., Saxe and Young 2016 for a review). These regions respond in both verbal (e.g., Fletcher et al. 1995; Saxe and Kanwisher 2003) and nonverbal (e.g., Gallagher et al. 2000; Jacoby et al. 2016; Saxe et al. 2006b) ToM paradigms, suggesting that they represent and process mental states in a language-independent format (see Adolphs 2009; Koster-Hale and Saxe 2013; Mar 2011 for reviews). On the other hand, language processing engages a set of regions on the lateral surfaces of left frontal and temporal lobes (e.g., Binder et al. 1997; Fedorenko et al. 2010). These regions are highly selective for meaningful and structured linguistic stimuli. They do not respond to a wide range of nonlinguistic stimuli/tasks, which have been proposed to share processing demands with language, including arithmetic processing, working memory, inhibitory control, and music (Fedorenko et al. 2011, 2012; Monti et al. 2012; see Fedorenko and Varley 2016 for a review). Importantly, they are also not engaged in processing broadly social stimuli, including nonlinguistic vocalizations, faces, biological motion, intentional actions, and speech-accompanying gestures (Deen et al. 2015; Jouravlev et al. 2018; Pritchett et al. 2018). This evidence en masse points to a neural dissociation between language and ToM.
Notwithstanding the evidence for distinct cognitive and neural mechanisms for ToM reasoning and language processing, there are reasons to postulate a deep connection between them. First, much of what people talk about either directly concerns mental states or requires mental state inference from information about the physical world (e.g., Dunbar 1994; Dunbar et al. 1997; Emler 1994; Feinberg et al. 2012). Second, most linguistic exchanges go beyond the literal meaning of the utterance and require pragmatic reasoning on the side of both the producer and comprehender, which involves ToM inference of communicative intentions (e.g., Benz et al. 2006; Clark and Wilkes-Gibbs 1986; Frank and Goodman 2012; Goodman and Frank 2016; Grice 1957, 1968, 1975; Sperber and Wilson 1986). Third, some aspects of language appear critical for the development of ToM abilities. For example, training children with no understanding of false beliefs on certain words or constructions allows them to pass tests of false belief understanding (e.g., Appleton and Reddy 1996; Clements et al. 2000; Hale and Tager-Flusberg 2003; Lohmann and Tomasello 2003; Slaughter and Gopnik 1996). And fourth, human language has been shown to be shaped by social communicative pressures to optimize information transfer at all levels, from the sound structure (e.g., Jakobson 1978; Ladefoged and Maddieson 1996; Maddieson and Disner 1984) to the lexicon (e.g., Blasi et al. 2016; Dautriche et al. 2016; Piantadosi et al. 2011; Zipf 1949) to grammar (e.g., Futrell et al. 2015; Gibson et al. 2013; Kirby 2000; Levinson 2016; Nowak and Krakauer 1999; Smith et al. 2003).
Given their intimate relationship, the ToM and language networks must have a way to interact with each other. Indeed, Deen et al. (2015) reported a small amount of overlap between language and ToM activations in left superior temporal cortex. However, another—possibly complementary—way to implement intersystem interaction is via synchronization in neural activity between the regions of one system and those of the other system (e.g., Cole et al. 2013). A large and growing recent literature (e.g., Bassett and Lynall 2013; De Luca et al. 2006; Power et al. 2011; Yeo et al. 2011) on correlations computed across voxels in the brain during naturalistic cognition (e.g., resting state) has identified a number of large-scale networks similar to those found with standard univariate contrasts. That is, regions that show similar activation profiles also tend to be correlated in “spontaneous fluctuations” of the fMRI signal at rest or during the processing of naturalistic stimuli (like listening to stories or watching movies). Hence, synchronization between brain regions is now a widely accepted signature of their functional integration (i.e., brain regions that are synchronized in their activity “work together” in the service of some perceptual, motor, or cognitive goal). Following the same reasoning, networks can be thought to show a degree of functional association if their fMRI signal fluctuations are correlated. Here, we use fMRI to ask whether the language and ToM networks exhibit such synchronization during naturalistic cognition.
METHODS
Participants
Fifty-five native English speakers (age 18–31, 15 men) from the Massachusetts Institute of Technology (MIT) and the surrounding Boston community participated for payment. Fifteen participants took part in experiment 1a (resting state). The same participants, plus one further participant, took part in experiment 1b (story comprehension). Ten different participants took part in experiment 2 (story comprehension, replication). Finally, 29 different participants took part in experiment 3 [story comprehension, replication with a verbal (3a) and nonverbal (3b) ToM localizer] (see Table 1).
Table 1.
Summary of the localizer and critical tasks included in each experiment
| Language Localizer | ToM Localizer—Verbal | ToM Localizer—Nonverbal | MD Localizer | Critical Task | |
|---|---|---|---|---|---|
| Experiment 1a (n = 15) | Yes | Yes | No | Yes | Resting state |
| Experiment 1b (n = 16; same as in experiment 1a + 1) | Yes | Yes | No | Yes | Story comprehension (5 stories each) |
| Experiment 2 (n = 10) | Yes | Yes | No | No | Story comprehension (4–8 stories each) |
| Experiment 3a (n = 29) | Yes | Yes (used in the analyses) | Yes | Yes | Story comprehension (1 story each) |
| Experiment 3b (n = 29) | Yes | Yes | Yes (used in the analyses) | Yes | Story comprehension (1 story each) |
| Exploratory experiment 3c (n = 15) | Yes | Yes (used in the analyses) | Yes | Yes | Dialogue comprehension |
| Exploratory experiment 3d (n = 14) | Yes | Yes (used in the analyses) | Yes | Yes | Low ToM content text comprehension |
ToM, theory of mind.
All participants in experiments 1 and 2 were right-handed, whereas five participants in experiment 3 were left-handed, as determined by the Edinburgh handedness inventory (Oldfield 1971). All of the left-handed participants showed typical left lateralization in the language localizer task described below. To determine lateralization, the number of language-contrast-activated voxels in the right hemisphere (RH) at a fixed significance threshold was subtracted from the number of language voxels in the left hemisphere (LH) at the same threshold, and the resulting value was divided by the sum of language voxels across hemispheres (see Mahowald and Fedorenko 2016).
All participants gave written, informed consent in accordance with the requirements of MIT’s Committee on the Use of Humans as Experimental Subjects, which approved the study protocol.
General Approach
The experiments were designed to assess the degree of synchronization between the language and ToM networks, as well as test whether this synchronization is specific to language and ToM and not a general property of any pair of large-scale networks (or any pair of networks that support high-level cognition, i.e., processes that have long been argued to be less modular than the perceptual/motor ones; e.g., Fodor 1983). Each participant completed a critical task (resting state and/or story comprehension), and two functional “localizer” tasks (e.g., Saxe et al. 2006a; Nieto-Castañón and Fedorenko 2012): a language network localizer (Fedorenko et al. 2010), and a ToM network localizer (Saxe and Kanwisher 2003) (Table 1). These localizers operationalize “language” and “ToM” more narrowly than the terms are sometimes used in the literature. Specifically, the language localizer targets higher-level aspects of language, including lexical and phrasal semantics, morphosyntax, and sentence-level pragmatic processing, to the exclusion of perceptual (speech or reading-related) and articulatory processes (see Fedorenko and Thompson-Schill 2014 for discussion). The ToM localizer targets “representational ToM” (Saxe 2006), akin to “cognitive ToM” (Dennis et al. 2013; Shamay-Tsoory et al. 2009), that is, inferences about the propositional content of other agents’ beliefs, desires, and goals, to the exclusion of “affective ToM,” roughly, the capacity to understand and empathize with others’ emotional states (e.g., Brothers and Ring 1992; Hein and Singer 2008; Singer and Lamm 2009).
Subsets of participants further completed one or two additional localizers: a localizer for the domain-general multiple demand (MD) network (Duncan 2010; Fedorenko et al. 2013), in experiments 1 and 3, and a nonverbal ToM localizer (Jacoby et al. 2016), in experiment 3 (Table 1). The MD network localizer was included to 1) allow for a replication of an earlier reported dissociation between the language and the MD network (Blank et al. 2014), in line with current emphasis in the field on replicability (e.g., Poldrack et al. 2017), and 2) assess the specificity of the language-ToM network association by testing the relationship between the ToM network and the MD network. In particular, including the MD network as a control ensures that observed within- and between-network synchronization is not global, due to an analysis artifact, and is not present for any pair of coherent, functionally integrated networks due to some degree of “background” connectivity among any pair of networks (or any pair of networks that support higher-level cognitive processes). The nonverbal ToM localizer served to exclude the possibility that language-ToM synchronization is due to the verbal nature of the main ToM localizer. Furthermore, the nonverbal ToM localizer plausibly engages a wider range of ToM-related processes, including aspects of affective ToM.
With the exception of the relatively recently developed nonverbal ToM localizer (Jacoby et al. 2016), all the other localizers have been extensively evaluated and validated in previous work, demonstrating that similar brain regions are robustly identified across variations in the specific materials, task, procedure, modality of presentation, and timing (e.g., Bruneau et al. 2012; Dodell-Feder et al. 2011; Fedorenko 2014; Fedorenko et al. 2010; Koster-Hale and Saxe 2013; Scott et al. 2016; Saxe and Kanwisher 2003; Saxe and Powell 2006; Saxe and Wexler 2005; Saxe et al. 2006b; Young et al. 2010). Furthermore, all three of the networks in question—the language network, the ToM network, and the MD network—appear to be recoverable from task-free resting state or other naturalistic cognition fMRI data (e.g., Blank et al. 2014; Tavor et al. 2016). As a result, although in any particular study a specific localizer contrast is used, we can be confident that the sets of regions we identify are not specific to that contrast given the prior methodological foundation behind each of the localizers. The localizers were used to define three sets of functional regions of interest (fROIs), separately in each individual brain. BOLD signal time-courses during the critical tasks were then extracted from these fROIs and their degree of synchronization across regions was measured, as detailed below.
Design, Stimuli, and Procedure
Language localizer task.
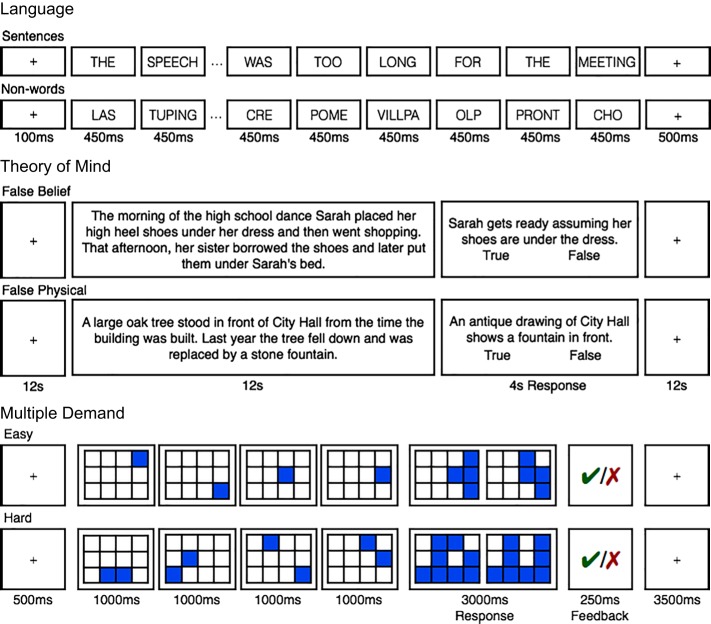
The task used to localize the language network is described in detail in Fedorenko et al. (2010) and targets brain regions that support high-level language processing, including both lexical-semantic and combinatorial (semantic and syntactic) processes (e.g., Bautista and Wilson 2016; Blank et al. 2016; Fedorenko et al. 2012). It also identifies right-hemisphere homologues of the classic, left-hemisphere language regions (e.g., Mahowald and Fedorenko 2016), which have been proposed to play a role in pragmatic reasoning (e.g., Coulson and Williams 2005; Diaz and Hogstrom 2011; Eviatar and Just 2006; Joanette et al. 1990; Kuperberg et al. 2000; Mashal et al. 2005). Briefly, we used a reading task that contrasted sentences (the critical condition) and lists of unconnected, pronounceable nonwords (the control condition; Fig. 1) in a standard blocked design with a counterbalanced order across runs (for timing parameters, see Table 2). By design, this localizer contrast subtracts out lower-level perceptual (speech or reading-related) and articulatory motor processes (see Fedorenko and Thompson-Schill 2014 for discussion). Stimuli were presented one word/nonword at a time. For the participants in experiment 2, each trial ended with a memory probe, and they had to indicate, via a button press, whether or not that probe had appeared in the preceding sentence/nonword sequence. The remaining participants read the materials passively (for these participants, we included a button-press task at the end of each trial, to maintain alertness). As noted above, this contrast has been shown to generalize across materials, task, and visual/auditory presentation (e.g., Braze et al. 2011; Fedorenko et al. 2010; Scott et al. 2016; Vagharchakian et al. 2012). Each participant completed between two and four runs. (A version of this localizer is available from https://evlab.mit.edu/funcloc/download-paradigms.)
Fig. 1.
Sample trials from the functional localizer paradigms. Language: sentences were contrasted with sequences of pronounceable nonwords. ToM: vignettes about false mental states were contrasted with vignettes about false physical states, each followed by a true/false statement (see Richardson et al. 2018 for screenshots from the nonverbal ToM localizer). MD: harder and easier versions of a spatial working memory task (location memory) were contrasted, each followed by a two-alternative forced choice question and feedback.
Table 2.
Timing parameters for the different versions of the language localizer task
| Version |
||||
|---|---|---|---|---|
| A | B | C | D | |
| Number of participants | 45 | 4 | 4 | 2 |
| Task: passive reading or memory? | PR | M | M | M |
| Words/nonwords per trial | 12 | 8 | 12 | 12 |
| Trial duration, ms | 6,000 | 4,800 | 6,000 | 6,000 |
| Fixation | 100 | 300 | 300 | 300 |
| Presentation of each word/nonword | 450 | 350 | 350 | 350 |
| Fixation | 500 | |||
| Memory probe | 1,350 | 1,000 | 1,000 | |
| Fixation | 350 | 500 | 500 | |
| Trials per block | 3 | 5 | 3 | 3 |
| Block duration, s | 18 | 24 | 18 | 18 |
| Blocks per condition (per run) | 8 | 4 | 6 | 8 |
| Conditions | Sentences Nonwords | Sentences Nonwords Word lists* | Sentences Nonwords Word lists* | Sentences Nonwords |
| Fixation block duration, s | 14 | 16 | 18 | 18 |
| Number of fixation blocks | 5 | 3 | 4 | 5 |
| Total run time, s | 358 | 336 | 396 | 378 |
| Number of runs | 2 | 3–4 | 2–3 | 2 |
M, memory; PR, passive reading.
Used for the purposes of another experiment; see Fedorenko et al. (2010).
ToM localizer task (verbal version).
The main paradigm used to localize the ToM network is described in detail in Saxe and Kanwisher (2003) and targets brain regions that support reasoning about others’ mental states. Briefly, the task was based on the classic false belief paradigm (Wimmer and Perner 1983) and contrasted verbal vignettes about false beliefs (e.g., a protagonist has a false belief about an object’s location; the critical condition) versus linguistically matched vignettes about false physical states (physical representations depicting outdated scenes, e.g., a photograph showing an object that has since been removed; the control condition). As noted above, this localizer focuses on ToM reasoning to the exclusion of affective or nonpropositional aspects of mentalizing. Participants read these vignettes, one at a time, in a long-event-related design with a counterbalanced order across runs. Each vignette was followed by a true/false comprehension question. Forty-seven participants completed two runs and eight completed one run due to time limitations, each lasting 272 s and consisting of five vignettes per condition. (A version of this localizer is available from http://saxelab.mit.edu/use-our-efficient-false-belief-localizer.)
ToM localizer task (nonverbal version).
The additional paradigm used to localize the ToM network in experiment 3b—based on a silent animated film—is described in detail in Jacoby et al. (2016; see also Richardson et al. 2018). Similar to the main ToM localizer, it targets brain regions that support inferences about others’ mental states, but, in contrast to the main localizer, it 1) is nonverbal, relying on participants engaging in mental state attribution from observed intentional actions, and 2) plausibly engages a broader range of mentalizing-related processes such as empathy. This nonverbal version was included to ensure that the reported language-ToM synchronization is not due to the verbal nature of the main ToM localizer. Briefly, the task consists of passive viewing of an animated short film, Partly Cloudy (Pixar Animation Studios), which contains sections likely to elicit mental state attribution, the “mental” condition (e.g., a character falsely believes they have been abandoned by a companion), as well as control sections which simply depict physical events, the “physical” condition (e.g., a flock of storks flying). The film was divided into sections, and each section was coded as the “mental” condition, the “physical” condition, or one of two other conditions, “pain” and “social,” by 5 independent coders. Jacoby et al. (2016) compared the activation patterns for the mental > pain contrast to those elicited by the verbal false belief ToM contrast described above and found that they are similar in individual subjects. Here we report results with the mental > physical contrast, which is conceptually more similar to our main localizer contrast. Twenty-nine participants completed a single run of this localizer, lasting 348 s, including four mental events with total duration 44 s and three physical events with total duration 24 s. (The localizer is available at http://saxelab.mit.edu/theory-mind-and-pain-matrix-localizer-movie-viewing-experiment the Partly Cloudy short film itself must be purchased from Pixar Animation Studios.)
MD localizer task.
The spatial working memory task used to localize the MD network is described in detail in Fedorenko et al. (2013; see also Blank et al. 2014) and targets brain regions sensitive to general executive demands (see Duncan and Owen 2000; Fedorenko et al. 2013; and Hugdahl et al. 2015, for evidence that diverse demanding tasks activate this network). On each trial, participants saw a 3 × 4 grid and kept track of eight (hard version; the critical condition) or four (easy version; the control condition) locations that were sequentially flashed two at a time or one at a time, respectively. Then, participants indicated their memory for these locations in a two-alternative, forced-choice paradigm via a button press. Feedback was provided after every trial. Hard and easy conditions were presented in a standard blocked design (4 trials in a 32-s block, 6 blocks per condition per run) with a counterbalanced order across runs. Each run included four blocks of fixation (16 s each) and lasted a total of 448 s. Thirty-three participants completed two runs and 12 completed one run due to time limitations. (This localizer is available from the authors upon request.)
Critical tasks.
In the resting state experiment, participants were instructed to close their eyes and let their mind wander but to remain awake while resting in the scanner for 5 min (the scanner lights were dimmed and the overhead projector was turned off). In the story comprehension experiments, participants listened to stories (5 stories each in experiment 1b; 4–8 stories in experiment 2; 1 story in experiment 3) over scanner-safe headphones (Sensimetrics, Malden, MA). Stories lasted between 4.5 and 6 min. They were adapted from existing, publicly available texts (fairy tales and short stories) (Futrell et al. 2017). The stories were recorded by two native English speakers (a male and a female). The stories were rich in mental state content (Table 3; the complete materials are available at http://github.com/languageMIT/naturalstories). In experiments 1b and 2, following the scan for each story, participants answered 12 (experiment 1b) or 6 (experiment 2) comprehension questions, presented in a two-alternative forced-choice format. For four participants, the behavioral data were lost due to equipment malfunction. For each of the remaining participants, accuracy on these questions was significantly above chance, as indicated by the binomial test (for all tests, P < 0.04; mean accuracy across participants: 82.22%, SD: 10.8). experiment 3 did not include comprehension questions.
Table 3.
Excerpts from three of the stories in the story comprehension task
| Examples | |
|---|---|
| The Bradford Boar | […] …the people of Bradford had second thoughts about visiting the well. That the people of Bradford bore the brunt of the beast’s ferocity was unfair in the eyes of the people of the region. […] By the handsome reward many felt tempted… […] Seeing the slain carcass of the boar the huntsman rejoiced in his good fortune. […] He was suddenly realizing his tricky situation could end badly for him. […] Listening to the tale, the Lord of the Manor tried to discern which huntsman was telling him the truth. […] |
| King of Birds | […] The eagle, who already thought himself the de facto king, arrived fashionably late. It was a hawk who was most excited about the meeting… […] It was decided that the bird that could fly the highest should be king. […] The little bird lay low near the eagle at first, but the eagle did not notice the bird hopping onto his back … […] … and he laughed to himself at how easy it had been to outwit the other birds. […] |
| Elvis | […] … I could not figure out what this Elvis Presley guy had that the rest of us boys did not have. […] When I got my new Buster Brown shoes, I was smiling from ear to ear. […] I cried desperately. […] … from his expression I could tell that he wished he could cut it as I had asked. […] “I am not a baby”, I said as I wiped the tears from my eyes. […] My head was no longer in the clouds. […] |
Marked in bold are a few examples of mental state content
Finally, we report the results of two exploratory conditions included for a subset of participants in experiment 3: a naturalistic dialogue (n = 15; experiment 3c) and a text about the life cycle of trees (adapted from Wikipedia), low in ToM content (n = 14; experiment 3d; Table 1). [Note that whereas experiments 3a and 3b differ in whether the verbal or the nonverbal ToM localizer was used in the analyses, experiments 3c and 3d differ in the nature of the critical conditions (see Table 1).] The former was included to assess whether language-ToM synchronization would generalize to naturalistic linguistic materials other than stories, which are nonetheless rich in mental state content and inferences; the latter was included to examine whether language-ToM synchronization would be attenuated for linguistic materials devoid of mental state content.
Data Acquisition and Preprocessing
Data acquisition.
Structural and functional data were collected on a whole-body 3 Tesla Siemens Trio scanner with a 32-channel head coil at the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT. T1-weighted structural images were collected in 176 axial slices with 1-mm isotropic voxels [repetition time (TR) = 2,530 ms; echo time (TE) = 3.48 ms]. Functional, BOLD data were acquired using an EPI sequence with a 90° flip angle and using GRAPPA with an acceleration factor of 2; the following parameters were used: thirty-one 4.4-mm-thick near-axial slices acquired in an interleaved order (with 10% distance factor), with an in-plane resolution of 2.1 mm × 2.1 mm, field of view in the phase encoding (A >> P) direction 200 mm and matrix size 96 mm × 96 mm, TR = 2,000 ms and TE = 30 ms. The first 10 s of each run were excluded to allow for steady-state magnetization.
Spatial preprocessing.
Data preprocessing was carried out with SPM12 and custom MATLAB scripts. Preprocessing of anatomical data included normalization into a common space (Montreal Neurological Institute template, resampling into 2-mm isotropic voxels, and segmentation into probabilistic maps of the gray matter, white matter, and cerebrospinal fluid ). Preprocessing of functional data included motion correction, normalization, resampling into 2 mm isotropic voxels, smoothing with a 4-mm FWHM Gaussian kernel and high-pass filtering at 200 s.
Temporal preprocessing.
Additional preprocessing of data from the resting state and story comprehension runs was carried out using the CONN toolbox (Whitfield-Gabrieli and Nieto-Castañon 2012) with default parameters, unless specified otherwise. Five temporal principal components of the BOLD signal time-courses extracted from the white matter were regressed out of each voxel’s time-course; signal originating in the cerebrospinal fluid was similarly regressed out. Six principal components of the six motion parameters estimated during offline motion correction were also regressed out, as well as their first time derivative. Next, as in Blank et al. (2014), the residual signal was bandpass filtered (0.008–0.09 Hz) to preserve only low-frequency signal fluctuations (Cordes et al. 2001). [As in Blank et al. (2014), the general pattern of results was similar without bandpass filtering (see also Gohel and Biswal 2015).]
Modeling localizer data.
For each localizer task, a general linear model estimated the effect size of each condition in each experimental run in each voxel. These effects were each modeled with a boxcar function (representing entire blocks/events) convolved with the canonical hemodynamic response function. The model also included first-order temporal derivatives of these effects, as well as nuisance regressors representing entire experimental runs and offline-estimated motion parameters. The obtained beta weights were then used to compute the functional contrast of interest: sentences > nonwords for the language localizer, false belief > false physical for the verbal ToM localizer, mental > physical for the nonverbal ToM localizer, and hard > easy spatial working memory for the MD localizer.
Defining functional regions of interest.
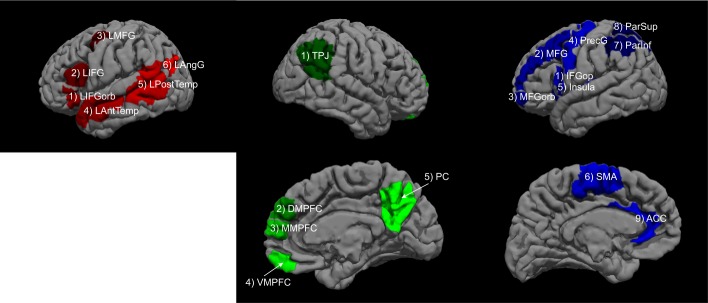
For each participant, functional ROIs were defined by combining two sources of information (Fedorenko et al. 2010; Julian et al. 2012): 1) the participant’s activation map for the relevant localizer contrast, and 2) group-level constraints (“masks”). The latter demarcated brain areas within which most or all individuals in prior studies showed activity for the localizer contrasts (Fig. 2).
Fig. 2.
Masks used to constrain the selection of subject-specific functional regions of interest in the three networks. Language (red): 1) LIFGorb, left inferior frontal gyrus, orbital portion; 2) LIFG; 3) LMFG, left middle frontal gyrus; 4) LAntTemp, left anterior temporal cortex; 5) LPostTemp, left posterior temporal cortex; 6) LAngG, left angular gyrus. ToM (green): 1) TPJ, temporoparietal junction; 2) DMPFC, dorsal medial prefrontal cortex; 3) MMPFC, middle medial prefrontal cortex; 4) VMPFC, ventral medial prefrontal cortex; 5) PC, posterior cingulate cortex and precuneus. MD (blue): 1) IFGop, IFG opercular portion; 2) MFG and 3) MFGorb, middle frontal gyrus and its orbital portion; 4) PrecG, precentral gyrus; 5) Insula; 6) SMA, supplementary motor area; 7) ParInf, inferior parietal cortex; 8) ParSup, superior parietal cortex; 9) ACC, anterior cingulate cortex.
For the language fROIs, we used masks derived from a group-level representation for the sentences > nonwords contrast in a set of 220 participants. These masks (available for download from http://web.mit.edu/evelina9/www/funcloc/funcloc_parcels.html) were similar to the masks derived from 25 participants, as originally reported in Fedorenko et al. (2010), and covered extensive portions of the left lateral frontal, temporal, and parietal cortices. In particular, six masks were used: three in the frontal lobe (LIFGorb, LIFG, LMFG), and three in the temporal and parietal cortices (LAntTemp, LPostTemp, and LAngG) (see glossary).
Although in the main analysis we were interested in the LH language regions, we additionally defined the RH homolog of the language network. To do so, the LH masks were mirror-projected onto the RH to create six homologous masks. By design, the masks (Fig. 2) cover large swaths of cortex to be able to accommodate interindividual variability. Hence the mirrored versions are likely to encompass RH language regions despite possible hemispheric asymmetries in the precise locations of activations (see Mahowald and Fedorenko 2016, for evidence). We included RH language regions because they have been argued to support some aspects of pragmatic/communicative processing (e.g., Coulson and Williams 2005; Diaz and Hogstrom 2011; Eviatar and Just 2006; Joanette et al. 1990; Kuperberg et al. 2000; Mashal et al. 2005). One may therefore hypothesize that RH language fROIs would show greater synchronization than LH language fROIs with the regions of the ToM network. We evaluated this hypothesis in one of the analyses.
For the ToM fROIs, we used masks derived from a group-level representation for the false belief > false physical contrast in an independent group of 462 participants (Dufour et al. 2013). These masks (available for download from http://saxelab.mit.edu/use-our-theory-mind-group-maps) included regions in the left and right temporoparietal junction, left and right precuneus/posterior cingulate cortex, and left and right dorsal, middle, and ventral medial prefrontal cortex, for a total of 10 regions (5 per hemisphere).
For the MD fROIs, following Fedorenko et al. (2013) and Blank et al. (2014), we used anatomical masks (Tzourio-Mazoyer et al. 2002) that correspond to brain regions linked to MD activity in prior work. These masks included regions in the opercular IFG, MFG, including its orbital part, insular cortex, precentral gyrus, supplementary and presupplementary motor area, inferior and superior parietal cortex, and anterior cingulate cortex, for a total of 18 regions (9 per hemisphere). [We note that functional masks derived for the MD network based on 197 participants, available at https://evlab.mit.edu/funcloc/download-parcels, overlapped closely with the anatomical masks used here; we chose to use the anatomical masks to facilitate comparisons between our functional data and those from Blank et al. (2014).]
These group-level masks, in the form of binary maps, were used to constrain the selection of subject-specific fROIs. In particular, for each participant, 12 language fROIs were created by intersecting the language masks with each participant’s unthresholded t-map for the sentences > nonwords contrast. For each participant and each mask, the 10% of voxels with the highest t values in the intersection image were chosen as the fROI (note that the voxels included in the RH fROIs were not constrained to be mirrored versions of their LH counterparts but were only constrained to land within a mirrored version of the masks). In a parallel fashion, 10 ToM fROIs and 18 MD fROIs were created by intersecting the ToM and MD masks with each participant’s unthresholded t-map for the false belief > false physical/mental > physical and hard > easy spatial working memory contrasts, respectively, and selecting the 10% of voxels with the highest t values within each mask. No contiguity constraints were imposed on the regions thus identified. In previous unpublished work we have observed that this approach to fROI definition (similar to the approach where all the voxels that pass a certain significance threshold are taken as the fROI) typically results in one large cluster with sometimes a few noncontiguous voxels and small clusters. Finally, because our focus was on intersystem synchronization, we excluded voxels that were included in more than one fROI due to (small amounts of) spatial overlap between activation maps (e.g., Deen et al. 2015; see Blank et al. 2014, for a similar approach). The overlap of the MD network with the language network (computed across the 45 participants in experiments 1 and 3) accounted for 2.07% of language fROIs and 0.69% of MD fROIs [mean (M) = 28.18 ; SD = 20.41 voxels, across participants]. The overlap of the MD network with the ToM network (computed across the same 45 participants, using the verbal ToM localizer) accounted for 0.42% of ToM fROIs and 0.15% of MD fROIs (M = 6.29; SD = 15.37 voxels). Finally, in line with Deen et al.’s (2015) results, the overlap between language and ToM (verbal version) activations was higher, but still small: overlapping voxels accounted for 7.93% of language fROIs and 7.26% of ToM fROIs (M = 107.75; SD = 52.96 voxels). These overlapping voxels were localized to the bilateral posterior temporal and parietal regions: PostTemp and AngG fROIs in the language network, and temporoparietal junction (TPJ) fROIs in the ToM network. (The overlap between language and the nonverbal version of the ToM localizer (computed across the 29 participants in experiment 3) was lower, accounting for 6.19% of language fROIs and 5.66% of ToM fROIs; M = 84.03; SD = 48.51 voxels.) No voxels overlapped across all three networks. On average (when considering all 55 participants), a total of 142.21 voxels per participant were excluded with the verbal ToM localizer and 120.41 with the nonverbal ToM localizer, comprising 2.05 and 1.73%, respectively, of the total number of voxels across the original fROIs.
The definition of fROIs as the 10% of voxels with the highest t values for a localizer contrast within a given mask balances the trade-off between 1) choosing only voxels that respond robustly to the relevant localizer contrast (sentences > nonwords, false belief > false physical/mental > physical, or hard > easy spatial working memory), and 2) having a sufficient number of voxels in the fROI. In addition, this procedure ensures that each fROI is constant in size across participants. Blank et al. (2014) compared this procedure to an alternative one, where fROIs were defined by intersecting language and MD masks with thresholded t-maps (at P < 0.001 uncorrected whole brain level) for the language and MD contrasts, and obtained similar results.
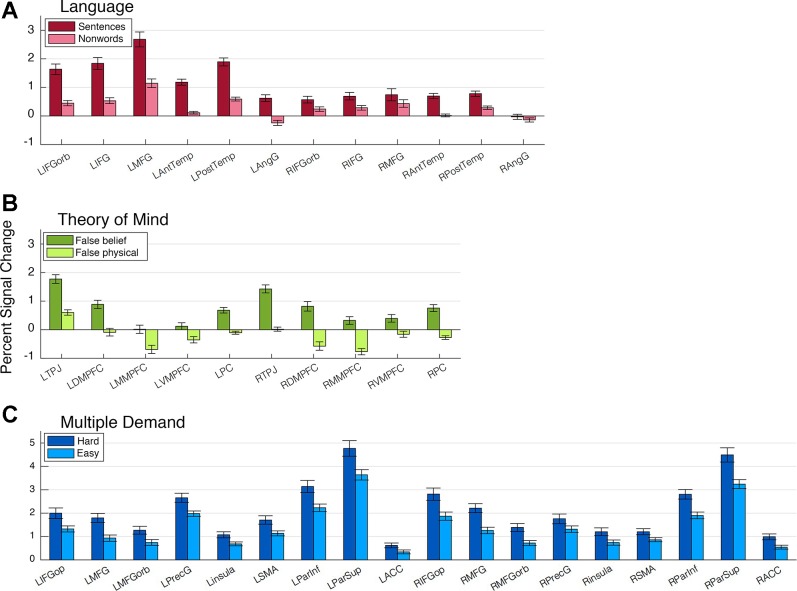
Before proceeding to the critical analyses, we ensured that the fROIs showed the expected functional signatures with respect to the localizer conditions (Fig. 3). To this end, the reliability of each localizer contrast effect, i.e., the difference between the regression coefficient estimates for its critical and control conditions, was tested via a twofold, leave-n-out cross-validation across runs: for each participant, we defined fROIs based on the even localizer run(s) and then derived independent estimates of the localizer contrast effect in these fROIs based on the odd run(s) and vice versa. The contrast effect estimates were averaged across the two partitions and tested for significance across participants (Bonferroni corrected for the number of regions within each network). Participants who only performed a single run of the MD localizer (n = 12) or a single run of the ToM localizer (n = 8) were excluded from this analysis, but their whole brain activation maps were visually examined to ensure that expected activation patterns obtained; the same was done for the activation maps for the mental > physical contrast in the nonverbal ToM paradigm, which consisted of a single run. The localizer effects were highly reliable in all fROIs except the RH AngG language fROI [t(54) = 2.05, not significant (ns) after Bonferroni correction; these and all reported t-tests were performed across participants; degrees of freedom are based on sample size]: LH language fROIs [t values(54) > 8.57, P values < 10−1], RH language fROIs excluding RAngG [t values(54) > 3.10, P values < 0.018), ToM fROIs [t(46) > 12.415, P values < 10−7], and MD fROIs [t values(32) > 5.075, P values < 10−4].
Fig. 3.
Responses of each network’s functional regions of interest (fROIs) to its localizer conditions. Bars correspond to percent signal change, relative to rest, in response to the target and control conditions of each localizer. The responses were estimated with twofold across-runs cross-validation, so that the data used for response estimation were independent from the data used for fROI definition. See glossary.
Critical functional correlation analysis.
For each fROI of each participant, we obtained a single BOLD signal time course by averaging the voxelwise time courses across all voxels. For each pair of fROIs, a Pearson’s product-moment correlation was computed between their time courses. These correlations were Fisher transformed to improve normality (Silver and Dunlap 1987), and the fROI-to-fROI correlation coefficients were averaged to get within-network correlations for each network and between-network correlations for each pair of networks. Note that this averaging, aimed at testing the key hypotheses concerning network interactions, was performed across the (Fisher-transformed) correlation coefficients, not across the fROI time courses. For within-network correlations, correlation-coefficient averages were computed across all pairs of the 6 LH language fROIs (15 pairs), 10 ToM fROIs (45 pairs), and 18 MD fROIs (153 pairs). In some analyses, we further include RH language fROIs (15 pairs) or all 12 LH and RH language fROIs (66 pairs). The same procedure was then repeated for between-network correlations. For the story comprehension condition, the resulting correlations were further averaged across stories within each participant before statistical testing.
To assay the patterns of functional correlations that characterize the networks and their potential interactions, we performed 1) a series of tests of difference from baseline; and 2) paired-sample t-tests. For all tests of difference from baseline, we compared the observed sample mean correlations against empirically estimated null distributions rather than performing conventional one-sample t-tests. Specifically, rather than estimating the probability that the observed sample means were drawn from a t-distribution defined by a mean of zero and n-1 degrees of freedom, we estimated the probability that they were drawn from a Gaussian null distribution with parameters (mean and standard deviation) estimated from the data (e.g., Blank et al. 2014; Lerner et al. 2011). Null parameters were estimated for each computed correlation: per fROI-to-fROI correlation, per participant and, in the story comprehension experiments, per story. For each pairwise correlation, the null estimation proceeded by Fourier transforming one fROI’s time course, shuffling the signal’s phases in the frequency domain (i.e., sampling uniformly with replacement from the signal phases and randomly assigning them to frequencies), transforming the signal back to the time domain, and correlating it with the intact time course of the second fROI (Theiler et al. 1992). This was repeated 1,000 times for each correlation, and the null mean and standard deviation were fitted to the resulting distribution of (Fisher-transformed) correlation coefficients. To perform group-level networkwise random effects tests, we aggregated the estimated null parameters across fROI pairs and across stories in the story comprehension experiments by averaging the means and pooling the standard deviations. The main advantage of this randomization approach is that it makes fewer assumptions about the sampling distribution than the conventional t-test; namely, it allows the parameters to be estimated from the data.
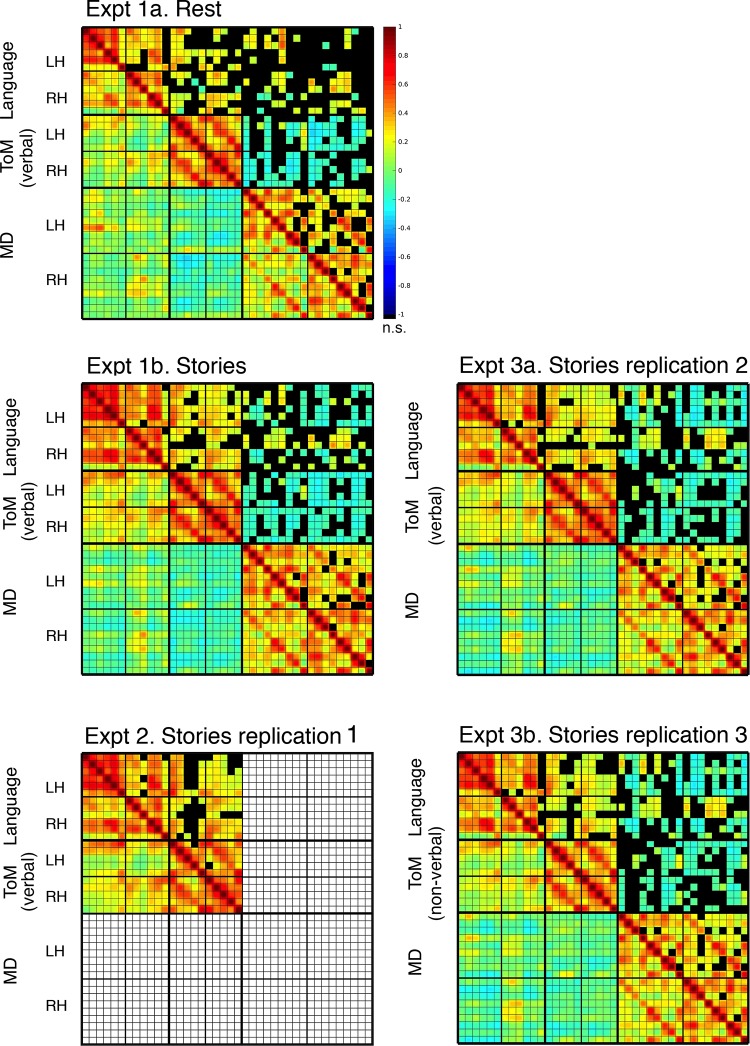
For visualization purposes, we also created five group-level matrices of fROI-to-fROI correlations, one for each experiment (Fig. 4). Specifically, the Fisher-transformed correlation between each pair of fROIs was averaged across participants (the Fisher transform decreases the bias in averaging; Silver and Dunlap 1987), and the resulting average correlations were then inverse Fisher transformed. However, to ensure that the patterns of functional correlations reported here were observed consistently across individual participants, all two-sample t-tests were performed over participant-level correlations. Only the hierarchical clustering analysis (see next section) relied on group-level average correlations.
Fig. 4.
Matrices for functional region of interest (fROI)-to-fROI correlations. The first column corresponds to experiments 1a (resting state), 1b (stories), and 2 [stories; multiple demand (MD) fROIs were not defined in that experiment because the MD localizer was not included]; the second column corresponds to experiments 3a [stories; theory of mind (ToM) fROIs defined by the verbal ToM localizer] and 3b (stories; ToM fROIs defined by the nonverbal ToM localizer). The half-matrix below the diagonal shows all correlations, the half-matrix above the diagonal highlights the significant ones (at P < 0.05, FDR corrected), with the nonsignificant ones colored in black. The order of the fROIs across rows and columns corresponds to the numbers used in Fig. 2; within each network, the regions are sorted by hemisphere (LH, left hemisphere; RH, right hemisphere). Qualitatively, these matrices illustrate our key findings: each of the three networks is internally integrated; the language and ToM network are further synchronized but dissociable. Neither the language nor the ToM network is correlated with the MD network.
Hierarchical clustering.
Hierarchical clustering was performed to examine whether the functional organization of the fROIs into networks could be recovered in a data-driven way, by asking whether, based on patterns of region-to-region correlations, 1) the regions would cluster together into their corresponding networks, and 2) the language and ToM networks would show a higher degree of similarity with each other than with the MD network.
Hierarchical clustering is an algorithm that creates a binary tree structure connecting elements in a set, such that the length of branches on the tree approximates the distances among the elements, as provided by the user (Hartigan 1975). The clustering together of elements, whose connecting path on the tree is shorter than a chosen length, therefore creates a partition of the element set without prespecifying the number of resulting clusters (in contrast to other common methods, such as k-means clustering). We performed hierarchical clustering on our fROIs, providing the group-level fROI-to-fROI correlation matrix as input so that the distance between two fROIs was defined as one minus their correlation. Clustering was based on average linkage so that two clusters were merged into a bigger cluster based on the mean distance between their respective members. So, at the lowest level of the tree, two fROIs were merged into a cluster if they had the highest correlation, i.e., smallest distance in correlation space, relative to all other pairs of fROIs. The distance of this new cluster from all remaining fROIs was then computed by averaging the two constituent fROIs’ distances from the remaining fROIs. The next merge occurred either between this new cluster and an fROI or between two other fROIs, depending on the new smallest distance. This was repeated until all fROIs were linked into a tree structure. The branch lengths in this structure represent the respective distances between fROI-fROI pairs, fROI-cluster pairs, or cluster-cluster pairs.
The optimal partition of fROIs, based on the resulting tree, was identified via a measure of modularity (Newman and Girvan 2004). First, by gradually decreasing the path length used as a criterion for clustering fROIs, we generated the set of all possible partitions licensed by our hierarchical clustering solution (the longest path length generates a single cluster consisting of all 40 fROIs; the shortest path length generates 40 singleton clusters). Then, for each partition, we computed a reformulated modularity measure that is appropriate for detecting clusters in correlated data (Gómez et al. 2009). High modularity values indicate clustering solutions where, within each cluster, the positive functional correlations are stronger (and the negative functional correlations are weaker) compared with what is expected under a null model. The null model is a random fROI-to-fROI correlation matrix that preserves, for each fROI, the sum of its positive correlations and the sum of its negative correlations with the other fROIs.
RESULTS
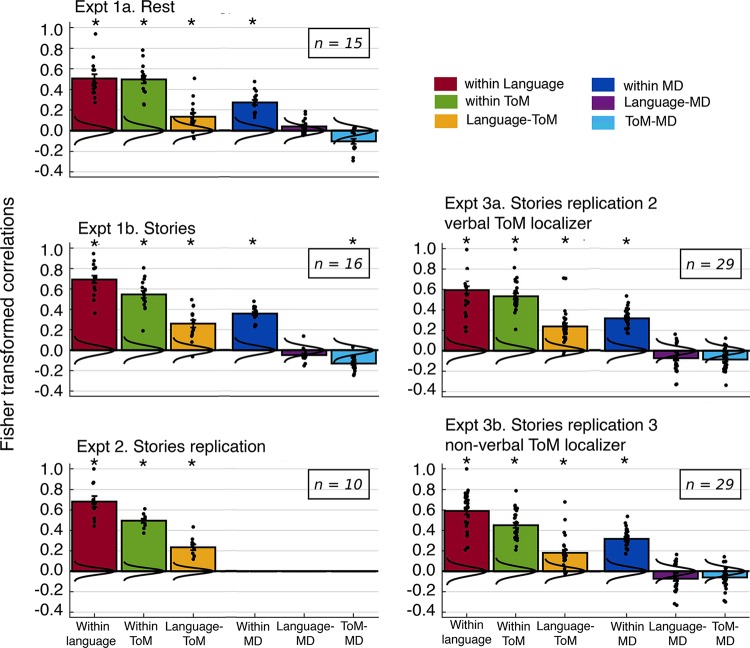
Figure 4 shows correlation matrices for the resting state (experiment 1a) and story comprehension (experiments 1b, 2, and 3a/b) conditions. The cells’ colors represent group-averaged Pearson’s correlations between pairs of language, ToM, and MD fROIs, within and across networks, computed on time courses of BOLD signal fluctuations. Prior to any networkwise statistical tests on these correlations, visual examination of the matrices already makes the key patterns clear. First, each of the three networks is internally integrated: pairs of fROIs within a network are strongly and positively correlated with each other. This replicates the pattern observed by Blank et al. (2014) for the language and MD networks and extends the findings to ToM fROIs: the ToM network is also characterized by low-frequency synchronization among its constituent regions, both at rest and during the processing of rich naturalistic stimuli (see also Buckner et al. 2008; Mason et al. 2008; Spreng et al. 2009; von dem Hagen et al. 2013; Yeo et al. 2011). Second, we replicate the dissociation between the language and MD networks reported by Blank et al. (2014): pairs of fROIs across these networks show uncorrelated activity both at rest and during story comprehension. Third and critically, many pairs of language and ToM fROIs show significant positive correlations, indicating some degree of functional association between the two networks. Furthermore, these correlations appear to be stronger during story comprehension. Importantly, the ToM network does not appear to be positively correlated with all large-scale brain networks. Specifically, the ToM and MD networks are not correlated, or slightly anticorrelated in their activity. These observations thus suggest a functional architecture comprised of three functionally separable networks, two of which—language and ToM networks—are characterized by some degree of functional synchronization. We now quantitatively evaluate these and some additional observations through a series of tests on the average pairwise correlations within and across networks.
The Internal Architecture of Each of the Three Networks
First, we tested the hypothesis that each of the networks is internally integrated by conducting one-sample t-tests on within-network correlations against empirical baselines, estimated via phase-shuffling of the BOLD time courses (see methods). We performed separate tests per hemisphere, as well as tests of the interhemispheric correlations for each network. The results, summarized in Table 4, show that all average within-network correlations are highly significant (P values < 10−6, Bonferroni-corrected for multiple comparisons, here and for all that follows; here, 9 comparisons for experiments 1a/b and experiment 3a/b, 6 comparisons for experiment 2, where the MD network was not included, see methods), consistent with the hypothesis that these networks are each internally integrated. To reduce the number of comparisons in the following analyses, we focus on the classical, left-hemisphere language fROIs (returning to the right hemispheric fROIs in Potential Hemispheric Differences in the Language-ToM Network Relationship, below), and collapse across LH and RH fROIs for the bilateral ToM and MD networks (6 comparisons for experiment 1a/b and experiment 3a/b, and 3 comparisons for experiment 2).
Table 4.
Within-network correlations per hemisphere and between hemispheres
| Experiment | Hemisphere | Language | ToM | MD |
|---|---|---|---|---|
| 1a. Rest | LH | 0.51 | 0.52 | 0.32 |
| (0.18) | (0.18) | (0.17) | ||
| RH | 0.37 | 0.56 | 0.36 | |
| (0.17) | (0.18) | (0.17) | ||
| LH-RH | 0.27 | 0.46 | 0.22 | |
| (0.17) | (0.18) | (0.17) | ||
| 1b. Stories | LH | 0.69 | 0.56 | 0.39 |
| (0.17) | (0.17) | (0.16) | ||
| RH | 0.47 | 0.59 | 0.42 | |
| (0.16) | (0.17) | (0.16) | ||
| LH-RH | 0.44 | 0.52 | 0.32 | |
| (0.16) | (0.17) | (0.16) | ||
| 2. Stories | LH | 0.68 | 0.48 | |
| (0.18) | (0.16) | |||
| RH | 0.44 | 0.59 | ||
| (0.16) | (0.17) | |||
| LH-RH | 0.37 | 0.46 | ||
| (0.16) | (0.16) | |||
| 3a. Stories | LH | 0.59 | 0.53 | 0.34 |
| (0.17) | (0.17) | (0.16) | ||
| RH | 0.36 | 0.60 | 0.38 | |
| (0.17) | (0.18) | (0.16) | ||
| LH-RH | 0.35 | 0.51 | 0.28 | |
| (0.16) | (0.17) | (0.16) | ||
| 3b. Stories (nonverbal ToM localizer) | LH | 0.59 | 0.43 | 0.34 |
| (0.17) | (0.17) | (0.16) | ||
| RH | 0.36 | 0.47 | 0.38 | |
| (0.17) | (0.17) | (0.16) | ||
| LH-RH | 0.35 | 0.44 | 0.27 | |
| (0.16) | (0.17) | (0.16) |
Numbers in parentheses are standard deviations. Correlations are highly significant (P < 10−6) within the three networks, at rest and during story comprehension. The language and multiple demand (MD) correlations replicate Blank et al. (2014). The within theory of mind (ToM) correlation is robust too, with the verbal and nonverbal ToM localizer.
The Relationship Between the Language and ToM Networks
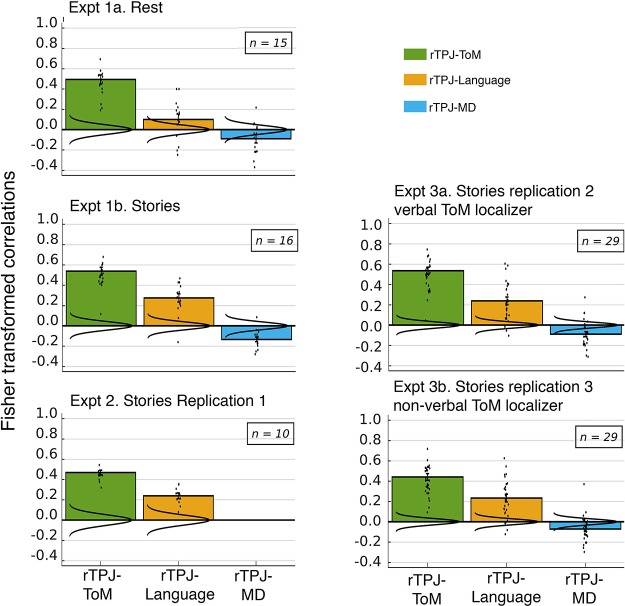
To test our critical hypothesis—that the language network and the ToM network are functionally associated—we tested the language-ToM correlation against baseline (Fig. 5). We observed a significant positive correlation, both at rest in experiment 1a (mean r = 0.135, SD = 0.15 across participants, P = 0.011) and during story comprehension in experiment 1b (mean r = 0.259, SD = 0.14, P < 10−9). The latter result held up in the two direct replications, experiment 2 (mean r = 0.235, SD = 0.09, P < 10−15) and experiment 3a (mean r = 0.238, SD = 0.16, P < 10−7). Visual inspection of the correlation matrices suggests that these effects are not driven by any one region or small subset of regions, and, importantly, that they are not driven by spatial proximity. For example, during story comprehension, the left TPJ fROI of the ToM network correlates not only with the nearby left posterior temporal language fROI, but with all LH language fROIs (and with most of the RH language fROIs). Similar results obtain for the cortical midline dorsal medial prefrontal cortex and PC ToM fROIs, which are not in the vicinity of any language fROIs. Perhaps most importantly, the results hold robustly for the right TPJ fROI of the ToM network, a region that has been argued to be most selective for mental state inference over other social inferences (e.g., Saxe and Powell 2006), as illustrated in Fig. 6.
Fig. 5.
Average within- and between-network correlations. Column 1 shows results from experiments 1a (resting state), 1b (stories), and 2 (stories). Column 2 shows experiment 3a [stories; theory of mind (ToM) functional regions of interest (fROIs) defined by the verbal ToM localizer], and 3b (stories; ToM fROIs defined by the nonverbal ToM localizer). Error bars are standard errors of the mean by participants. Black dots correspond to the individual participants’ values. Vertical curves are Gaussian fits to empirical null distributions. Significant correlations (Bonferroni-corrected within each experiment; see methods) are marked with asterisks.
Fig. 6.
Average correlations between the temporoparietal junction (rTPJ) theory of mind (ToM) functional region of interest (fROI) (the most mentalizing-selective component of the ToM network; Saxe and Powell 2006) and the rest of the ToM network, the left hemisphere (LH) language network, and the multiple demand (MD) network. The same convention is followed as in Fig. 5.
The language-ToM synchronization replicated when the ToM fROIs were defined by the nonverbal ToM localizer in experiment 3b (mean r = 0.181, SD = 0.15, P < 10−4). Although the observed synchronization is robust and only slightly lower in magnitude than the one observed with the verbal-ToM-defined fROIs, this difference is significant in a paired-sample t-test [t(28) = 3.613, P = 0.004]. Notably, however, the correlation within the ToM network is also significantly lower when the fROIs are defined by the nonverbal ToM localizer [t(28) = 3.96, P = 0.001; no other within- or between-network correlations differ significantly]. At least two explanations are possible. First, the nonverbal ToM localizer may be less robust or less reliable at identifying ToM-responsive voxels. And second, there may be a genuine difference between the fROIs defined by the verbal versus nonverbal ToM localizer in their preference for mental content. In particular, the former may be better tuned to linguistically packaged mental content, leading to greater synchronization with the language fROIs. Following this reasoning, the fROIs defined by the nonverbal ToM localizer may be better tuned to mental content inferred from observable actions and may thus be synchronized to some degree with the action observation system (e.g., Grosbras et al. 2012). More research is needed to resolve the source of this difference. But crucially for the purposes of the current study, reliable language-ToM synchronization is observed regardless of whether the ToM fROIs are defined by the verbal or nonverbal paradigm.
In addition, replicating Blank et al. (2014), the language-MD correlations did not significantly differ from baseline (rest, experiment 1a: mean r = 0.042, SD = 0.069, ns; stories, experiment 1b: mean r = −0.047, SD = 0.062, ns; story replications 2 and 3, experiment 3a/b: mean r = −0.072, SD =0.124, ns). It is important to note that this difference between the language-ToM correlation and the language-MD correlation is not due to interregion distances being, on average, shorter for pairs of language and ToM regions than for pairs of language and MD regions: in fact, interregion distances are quite similar (language-ToM average distance between the mask centroids: 85.24 mm, language-MD average distance: 81.19 mm; t(334) = 1.18, P = 0.24).
Finally, the ToM network and the MD network were marginally anticorrelated at rest (experiment 1a: mean r =−0.104, SD = 0.102, P = 0.085) and significantly anticorrelated during story comprehension (experiment 1b: mean r = −0.129, SD = 0.07, P = 0.006), but the latter effect was not robust: it did not replicate in experiment 3a (mean r = −0.085, SD = 0.103, ns) or experiment 3b, with nonverbal-localizer-defined ToM fROIs (mean r = −0.06, SD = 0.1, ns), where the ToM-MD correlation was not different from zero.
In our data set [cf. the earlier Blank et al. (2014), investigation of the language-MD relationship], we observed somewhat lower within-network integration for the MD network, compared with the language and the ToM network. To ensure that this greater heterogeneity within the MD network is not responsible for the lack of reliable ToM-MD correlations, we performed a control analysis using data from experiment 3a. We chose a subset of six MD fROIs (bilateral insula, supplementary motor area, and anterior cingulate cortex), whose average interregion correlation (r = 0.58; cf. r = 0.32 for the entire MD network in the same data set) closely matched the within-language (r = 0.59) and within-ToM (r = 0.53) correlations. We then examined this MD cluster’s correlation with the language and ToM networks. The pattern did not differ from that obtained with the entire MD network: weakly negative correlations with the language (r = −0.09) and ToM (r = −0.05) networks.
Next, we tested whether the language and ToM networks are dissociable despite their significant positive internetwork correlations, by performing paired-sample t-tests comparing the within-network language and ToM correlations against the between-network language-ToM correlation (Fig. 5). In both the resting state and story comprehension conditions, we found significantly lower language-ToM correlations than within-language correlations [rest, experiment 1a: t(14) = 6.836, P < 10−4; stories, experiment 1b: t(15) = 14.419, P < 10−9] and within-ToM correlations [rest: t(14) = 7.149, P < 10−5; stories: t(15) = 6.99, P < 10−5]. The same pattern was observed in the story comprehension replications in experiments 2 and 3, for both the within-language versus language-ToM correlations [experiment 2: t(9) = 11.265, P < 10−5; experiment 3a: t(28) = 11.656, P < 10−11; and experiment 3b: t(28) = 14.46, P < 10−13] and the within-ToM versus language-ToM correlations [experiment 2: t(9) = 8.705, P < 10−4; experiment 3a: t(28) = 14.826, P < 10−13; and experiment 3b: t(28) = 13.37, P < 10−12]. Therefore, although the language and ToM networks appear to be functionally coupled, they remain dissociable both at rest and during story comprehension.
These key findings were reproduced with two alternative statistical approaches: 1) a permutation test, in which fROIs were randomly assigned to be “language” or “ToM” fROIs to obtain an empirical null distribution of the correlation coefficients, and 2) a linear mixed effects model with random intercepts and slopes per fROI and per participant (Bates et al. 2008).
Finally, we tested whether the language-ToM synchronization was reliably stronger during story comprehension compared with rest in a set of participants in experiment 1a who were also tested in experiment 1b (n = 15) (Fig. 5). (Note that although experiment 1b, the stories condition, has more data than experiment 1a, the rest condition, the amount of data should not affect the observed sizes of the correlations, only the within-subject reliability of the estimates. As a result, this comparison is reasonable.) Indeed, the language-ToM correlation was significantly higher during story comprehension [t(14) = 2.956, P = 0.031], suggesting that the two networks increase online coordination during the processing of rich naturalistic stimuli, perhaps especially stimuli that place high demands on both linguistic and social processing. Interestingly, the increase in online coordination during the processing of such stimuli is not a general property of every two high-level networks. In particular, the language-MD and ToM-MD correlations actually decreased between rest and story comprehension, significantly for the language-MD correlation [t(14) =−3.724, P = 0.006], and numerically for the ToM-MD correlation [t(14) = −0.875, ns). When looking at differences in the within-network correlations between rest and story comprehension, we observed a significant increase for the language and the MD network during story comprehension [t(14) = 3.428, P = 0.012, and t(14) = 3.530, P = 0.01], but not for the ToM network [t(14) = 1.717, ns].
Potential Hemispheric Differences in the Language-ToM Network Relationship
We have so far focused on the classic LH language network, given that the LH language regions have been causally implicated in language processing, with damage to these regions in adulthood resulting in deficits in language production and/or comprehension (e.g., Bates et al. 2003; Fridriksson et al. 2016; Mesulam et al. 2015; Mirman et al. 2015; Penfield and Roberts 1959; Whitaker and Ojemann 1977). However, the RH homolog of the language network has received a lot of attention in the literature over the years, and in ways relevant to the question investigated in the current study. In particular, RH language regions have been implicated in some aspects of nonliteral language processing and pragmatic reasoning, which involves the computation of speakers’ intended meanings (e.g., Coulson and Williams 2005; Eviatar and Just 2006; Joanette et al. 1990; Kuperberg et al. 2000; Mashal et al. 2005). One might therefore hypothesize that these regions would have an even stronger relationship with the ToM network than the LH language regions (see also Chai et al. 2016). To test this hypothesis, we compared the LH and RH language networks (Fig. 7). At rest, the average correlation of the RH language fROIs with the ToM network did not significantly differ from that of the LH language fROIs [t(14) = 0.886, ns]. During story comprehension, the average correlation of the RH language fROIs with the ToM network either did not significantly differ from that of the LH language fROIs [in experiment 2: t(9) = −0.319, ns; and in experiment 3b: t(28) = 1.421, ns] or was actually weaker [in experiment 1b: t(15) = 2.492, P = 0.025; and in experiment 3a: t(28) = 2.147, P = 0.041) (Fig. 7; only results for experiments 1a/b and 2 are shown). Thus, we do not find evidence of a stronger connection between the RH language regions and the ToM network (compared with the LH language regions and the ToM network).
Fig. 7.
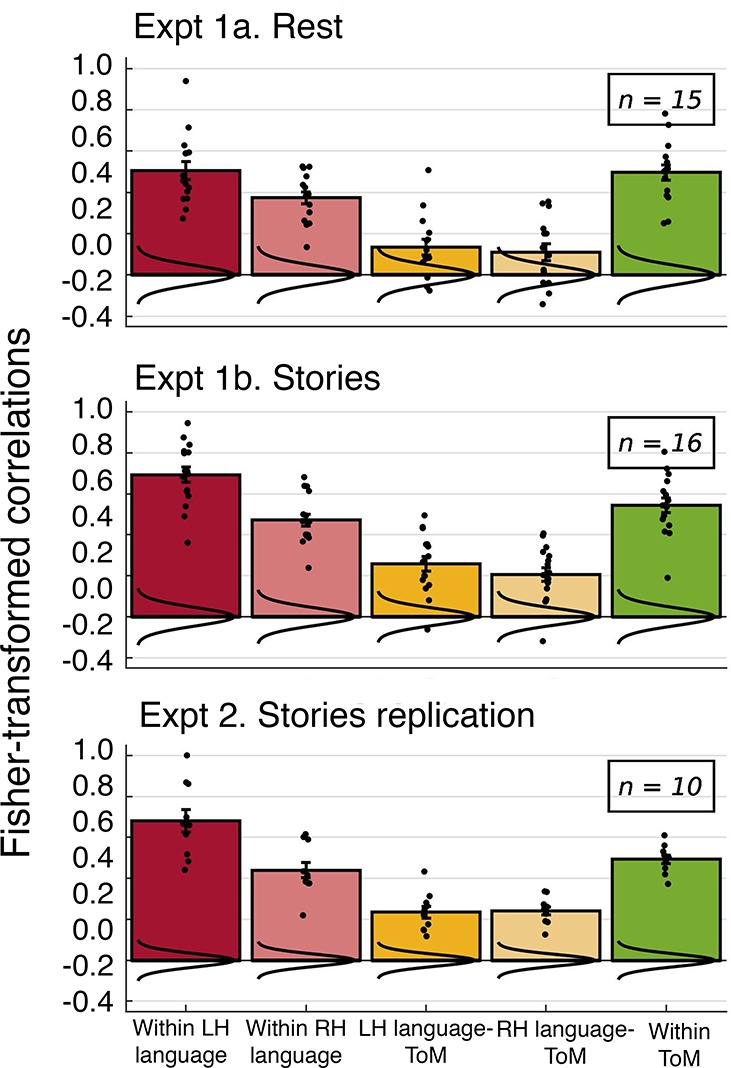
Comparison of between-network correlations for left hemisphere (LH) language regions and the theory of mind (ToM) network (bright yellow) versus right hemisphere (RH) language regions and the ToM network (pale yellow) for experiments 1a (rest), 1b (stories), and 2 (stories). The bright red and green bars show the same data as shown in Fig. 5. The pale red bar shows the average within-network correlation for the RH language functional regions of interest (fROIs). Error bars are standard errors of the mean by participants. Black dots correspond to the individual participants’ values. Vertical curves are Gaussian fits to empirical null distributions. The hypothesis that RH language regions play a special role in pragmatic, ToM-based inference predicts stronger correlations between RH language regions and the ToM network (compared with the LH language regions and the ToM network). This prediction was not supported in any of our experiments.
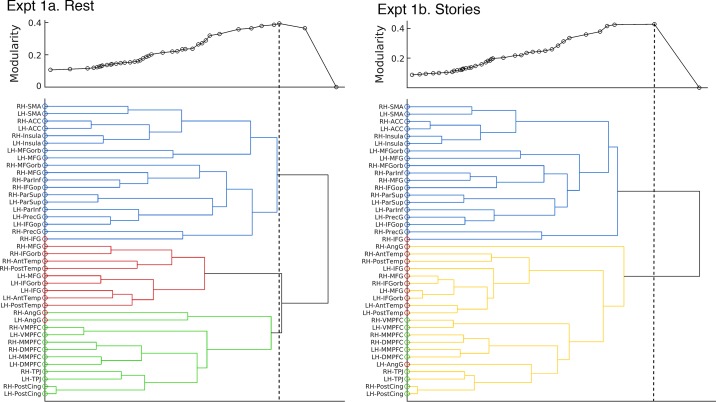
Hierarchical Clustering
The tree structures (dendrograms; Fig. 8) constructed from the group-level fROI-to-fROI correlations recapitulate the results presented so far, but using a data-driven approach (in contrast to the hypothesis-driven grouping of fROIs into predefined networks in the previous sections). The hierarchical structure discovered in the data is dominated by the division into functional networks: in both the resting state and story comprehension conditions, the topmost partition separates out the MD fROIs from the ToM and language fROIs, with the exception of the RH IFG language fROI, which is assigned to the MD network cluster; one branch below, within the language-ToM cluster, segregation between language and ToM regions is mostly maintained, with the exception of the AngG language fROIs, which cluster with the ToM network, bilaterally at rest, and only the LH AngG during story comprehension (see also Blank et al. 2014; Chai et al. 2016; Mahowald and Fedorenko 2016; Mineroff et al. 2018, for additional evidence that the AngG language fROIs pattern differently from the rest of the language network). Hence, here too, we observed a tripartite organization into functional networks, where language and ToM fROIs are more closely related to each other than to MD fROIs. The modularity metric (see methods) supports the hypothesis that the dominant organizing principle is that of functional specialization. Modularity peaked at two clusters in experiments 1b (shown in Fig. 8) and 3a, story comprehension with verbal ToM localizer, or at three clusters in experiments 1a, resting state (shown in Fig. 8) and 3b, story comprehension with nonverbal localizer.
Fig. 8.
Results of hierarchical clustering for experiment 1a, resting state (left) and experiment 1b, story comprehension (right). Hierarchical clustering creates a binary tree where branch length (horizontal lines) corresponds to the similarity (here, average correlation across participants) between functional regions of interest (fROIs). The color of the dots on the y-axis represents our a priori assignment of fROIs to networks: multiple demand (MD; blue), language (red), and theory of mind (ToM; green). Above each tree diagram, modularity is plotted for all fROI partitions in the tree. Each point in the modularity plot corresponds to a partition: an imaginary vertical line drawn from a location where two fROIs or clusters of fROIs connect to form a higher-level cluster. The dashed lines represent the points of maximum modularity, which correspond to a partition into networks. During resting state, maximum modularity is at a partition into three clusters: MD (blue lines), language (red lines), and ToM (green lines). All fROIs are correctly assigned to clusters, apart from the right IFG language fROI at rest, assigned to the MD network and the bilateral angular gyrus (AngG) language fROIs which were assigned to the ToM network, despite being defined with the language localizer. During story comprehension, maximum modularity is at a two-partite division: an MD cluster (blue) and a language-ToM cluster (yellow). Within the latter, fROIs mostly remain segregated into language and ToM fROIs, except for the left angular gyrus (AngG) language fROI. See glossary.
Exploratory Experiments: Dialogue and Low-ToM-Content Text
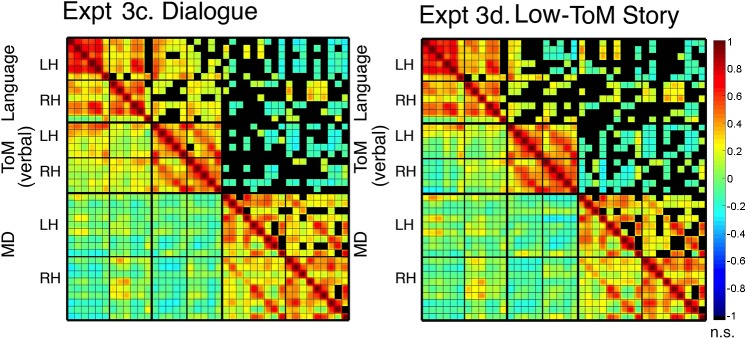
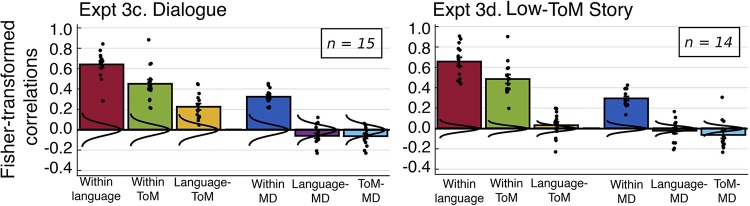
Lastly, we report the results of two exploratory conditions included in experiment 3: first, an extension of the story comprehension results to a dialogue, i.e., another naturalistic linguistic stimulus rich in mental state attribution (experiment 3c), and, second, an assay of whether the language-ToM synchronization may be attenuated during the processing of a linguistic stimulus low in mental state attribution (experiment 3d) (Figs. 9 and 10). In both conditions, the internal synchronization of all networks was maintained: within-language (experiment 3c: r = 0.643, SD = 0.127, P < 10−30; experiment 3d: r = 0.658, SD = 0.158, P < 10−100), within-ToM (experiment 3c: r = 0.451, SD = 0.171, P < 10−16; experiment 3d: r = 0.486, SD = 0.165, P < 10−69), and within MD (experiment 3c: r = 0.325, SD = 0.072, P < 10−9; experiment 3d: r = 0.295, SD = 0.08, P < 10−27), and the language and MD networks were again dissociated (experiment 3c: r = −0.058, SD = 0.094, ns; experiment 3d: r = −0.021, SD = 0.108, ns). Critically, in the dialogue condition, the language-ToM synchronization was preserved (r = 0.227, SD = 0.121, P < 10−4), though the networks remained dissociable in paired sample t-tests (within language versus language-ToM: t(14) = 12.428, P < 10−7; within ToM versus language-ToM, t(14) = 7.748, P < 10−5). Intriguingly, however, for the low-ToM-content stimulus, the language-ToM correlation did not significantly differ from baseline (r = 0.035, SD = 0.119, ns), suggesting strong context sensitivity of the synchronization (cf. Gratton et al. 2018). The same patterns held when the nonverbal ToM localizer was used for ToM fROI definition (results not shown).
Fig. 9.
Matrices for functional region of interest (fROI)-to-fROI correlations for the two exploratory conditions: experiment 3c, dialogue and experiment 3d, low-ToM-content text. Same convention is followed as in Fig. 4 as well as the same statistical procedure for determining the significance of correlations. Qualitatively, these matrices illustrate that the resting state and story comprehension results extend to another naturalistic linguistic stimulus rich in mental state attribution (experiment 3c), whereas a naturalistic stimulus low in mental state content does not elicit reliable language-theory of mind (ToM) synchronization (experiment 3d).
Fig. 10.
Average within- and between-network correlations for the two exploratory conditions: experiment 3c, Dialogue and experiment 3d, low-theory of mind (ToM)-content text. The same convention is followed as in Fig. 5.
It is striking that the language-ToM synchronization is weaker during the processing of the low-ToM-content stimulus compared with rest. One speculative explanation may be that at rest people tend to engage in ToM reasoning, whereas the cognitive demands of processing the low-ToM-content stimulus divert attention away from such processing. These exploratory results warrant further investigation.
DISCUSSION
Across three fMRI experiments (n = 55 participants total), we examined the relationship among three large-scale networks in the human brain: the networks that support language processing, social cognition (the Theory of Mind, or ToM, network), and executive functions (the multiple demand, or MD, network). We found that the language and ToM networks show synchronized activity at rest and during story comprehension. This finding suggests a degree of functional integration between these otherwise dissociable sets of cortical regions. Importantly, the observed synchronization 1) was selective (neither network correlated with signal fluctuations in the domain-general MD network) and 2) was robust to variation in the definition of the ToM network with a verbal versus a nonverbal paradigm.
Language and ToM are deeply interconnected aspects of human cognition, which possibly coevolved (e.g., Hurford et al. 1998; Malle 2002; Pinker 2010; Woensdregt and Smith 2017). For example, according to some evolutionary accounts, increases in social complexity led to the increase in general intelligence as well as the emergence of a robust communication system in the form of language (e.g., Borrego 2017; Borrego and Gaines 2016; Dunbar and Shultz 2007; Lihoreau et al. 2012; Reader and Laland 2002; Southgate et al. 2010). This view finds support in the literature on child and adult language processing, where successful comprehension has been argued to require access to knowledge of the mental state of the conversation partner (e.g., Brown-Schmidt et al. 2008; Heller et al. 2008; Nadig and Sedivy 2002; Southgate et al. 2010).
According to other accounts, the increasing sophistication of our communication skills has allowed for richer mental state inferences (e.g., Astington and Baird 2005; de Villiers and de Villiers 2014; Heyes and Frith 2014). This view finds support in the developmental literature: acquiring certain words or constructions appears to be critical for the development of mentalizing abilities. Knowledge of mental state verbs and sentential embedding correlate with success on false belief tasks (e.g., Astington and Baird 2005; Astington and Jenkins 1999; De Villiers 2000; Dunn et al. 1991; Milligan et al. 2007; Watson et al. 2001; Wellman et al. 2001), and training children who do not pass false belief tasks on the relevant words/constructions allows them to pass those tasks (e.g., Appleton and Reddy 1996; Clements et al. 2000; Hale and Tager-Flusberg 2003; Lohmann and Tomasello 2003; Slaughter and Gopnik 1996). This causal relationship is also observed in deaf individuals born to hearing parents, who often lack linguistic input during early childhood, and who exhibit delays in ToM development (e.g., De Villiers 2005; Peterson and Siegal 2000; Pyers and Senghas 2009; Schick et al. 2002, 2007; Woolfe et al. 2002). Furthermore, these delays are associated with delayed specialization of the ToM network for mental state processing (Richardson et al. 2018).
Despite this deep connection, the computational demands of language processing and mentalizing are different (cf. Goodman and Stuhlmüller 2013; Sperber and Wilson 2002). Language comprehension requires us to interpret linguistic signals with respect to our knowledge of meaning-form mappings, and language production requires us to select the relevant linguistic forms to convey the intended meanings (e.g., Goldberg 1995; Jackendoff 2002). Our linguistic knowledge is plausibly stored within the left-lateralized language network, which responds selectively during language processing (e.g., Fedorenko et al. 2011; Monti et al. 2012; see Fedorenko and Varley 2016 for a review). In contrast, mentalizing requires us to map observed behaviors onto invisible mental states and to predict behavior from attributed mental states (e.g., Dennett 1978; Premack and Woodruff 1978; Wellman 1979, 1985). These computations appear to be supported by a bilateral network, which includes regions in the temporoparietal junction and along the cortical midline (e.g., Fletcher et al. 1995; Gallagher et al. 2000; Saxe and Kanwisher 2003; Saxe and Wexler 2005). And yet, given that most linguistic exchanges concern our own or others’ mental states (e.g., Dunbar 1994; Dunbar et al. 1997; Emler 1994; Feinberg et al. 2012), and go beyond the literal meaning of utterances (e.g., Benz et al. 2006; Frank and Goodman 2012; Goodman and Frank 2016; Grice 1957, 1968, 1975; Sperber and Wilson 1986), the language and the ToM networks must have a way to share information.
Deen et al. (2015) reported small overlap between language processing and mental state reasoning within superior temporal cortex. As Deen et al. acknowledge, neural overlap is ambiguous: it can reflect 1) engagement of the relevant region in multiple cognitive processes, perhaps in a context-dependent fashion, similar to the regions of the domain-general MD network (e.g., Asaad et al. 2000; Duncan et al. 2000; Duncan and Owen 2000; Fedorenko et al. 2013; Friedman and Miyake 2000; Hugdahl et al. 2015; Mitchell et al. 2016; Miyake et al. 2001; Wallis et al. 2001), 2) a shared computation, or 3) some kind of integration process that combines information from multiple domains (e.g., Cole et al. 2010). More work is needed to distinguish among these interpretations. Here, we focused on another, possibly complementary, way in which the language and ToM networks could communicate: synchronization of neural fluctuations, after removing the small number of overlapping voxels (e.g., Buckner et al. 2013; Engel et al. 2001; Fries 2005; Fries et al. 2001; Gray and Singer 1989). We examined such fluctuations during naturalistic cognition—resting state (experiment 1a) and story comprehension (experiments 1b, 2, and 3)—and observed reliably synchronized activity. (The two networks were still robustly dissociable, as evidenced by higher within-network compared with between-network correlations.)
The internetwork synchronization is intriguing, especially given its selectivity: the language network is not synchronized with the domain-general MD network (Blank et al. 2014, replicated here in experiments 1 and 3), despite its alleged role in some aspects of language processing (see Fedorenko 2014 for a review); and the ToM network, similarly, is not synchronized with the MD network, despite the high executive demands of certain ToM tasks (e.g., Carlson and Moses 2001; Carlson et al. 2015; Lewis and Osborne 1990; Powell and Carey 2017; Roth and Leslie 1998; Rubio-Fernández and Geurts 2013, 2016; Wellman et al. 2001; cf. Saxe et al. 2006b). The lack of correlations with the MD network shows that not all networks that support high-level cognition are synchronized. It is, however, possible, perhaps even likely that either or both the language and ToM networks are correlated with some other networks. For example, as suggested above, the ToM network may be correlated with action observation regions, at least during understanding of intentional actions; and the language network may be correlated with, say, music-selective regions (Norman-Haignere et al. 2015) during the processing of songs. These predictions remain to be empirically evaluated. We cannot at present explain the lack of correlations between the MD network and the ToM or language networks. The relationship between language and ToM may be special. But it is also possible that the language and ToM networks interact with the MD network but by means other than synchronization, or perhaps, via transient synchronization that does not translate to fMRI signal synchronization. Our results call for future work to examine these possibilities.
What do language-ToM correlations reflect? Prior studies have suggested that functional correlations reflect anatomical connectivity and/or history of coactivation (e.g., Deco and Corbetta 2011; Deco et al. 2011, 2013; for reviews and additional accounts, see He et al. 2008; Keller et al. 2011; Matsui et al. 2011; Schölvinck et al. 2010; Shmuel and Leopold 2008; Warren et al. 2017). Whether the internetwork correlations reported here correspond to anatomical connections remains to be discovered. The coactivation history account seems a priori plausible given that language processing and mentalizing are often engaged simultaneously in our daily life. However, it will be important in future work to characterize the precise conditions under which neural activity in both networks increases. One way to do so would be to further probe the degree and nature of stimulus/task-dependence of the language-ToM correlations. In the current study, story comprehension elicited somewhat stronger internetwork synchronization than resting state. More strikingly, in the exploratory experiment 3d, in which participants listened to a text low in mental state content, no correlation between the language and ToM networks was observed. This stimulus/task dependence is contra recent evidence that the low-frequency fluctuations that are typically examined in naturalistic-cognition fMRI paradigms are highly stable within individuals with little modulation by stimulus or task (Gratton et al. 2018; Lynch et al. 2018). Perhaps the greater sensitivity afforded by the individual-subject functional localization approach (e.g., Nieto-Castañón and Fedorenko 2012) is enabling us to detect these effects. In any case, given our findings, an important direction for future work is to systematically vary different properties of naturalistic stimuli (e.g., linguistic versus nonlinguistic, or high versus low in mental state content) to examine the effects of those properties—formally specified or empirically estimated using behavioral paradigms—on the strength of language-ToM correlations. Another way to shed light on this question could be to identify the time periods in the naturalistic stimuli that show the highest internetwork correlations. These time periods could be analyzed for their linguistic/social features, and specific hypotheses could then be generated about the causes for the synchronization increases, and subsequently tested using new materials.
It is worth noting that that neither anatomical connectivity nor history of coactivation can explain the observed stimulus/task dependence of the language-ToM correlations. Both accounts predict stable correlations across variations in stimuli. The reason stimulus dependence poses a challenge for interpreting functional correlations is that these correlations are driven by very low frequencies in the fMRI signal, around one cycle every 10 s (0.1 Hz) (Biswal et al. 1995; Fox and Raichle 2007; Greicius et al. 2003). Although some cognitive processes do unfold over such slow time scales, including potentially aspects of ToM-related discourse processing (e.g., Lerner et al. 2011), this rate of fluctuations is too slow to be generally driven by neuronal activity (e.g., Fries et al. 2001). Recent findings suggest that slow, long-range functional correlations may be driven by functionally relevant hemodynamic processes (Mateo et al. 2017), but these results only provide preliminary clues for the underlying mechanisms. Notwithstanding the still limited understanding of the mechanisms and exact functions of slow, long-range connectivity changes, it is clear that 1) region-to-region “allegiances” in fMRI synchronization change on the order of seconds to minutes (Bassett et al. 2013; Whitlow et al. 2011), and 2) these changes have functional significance (Bassett et al. 2011). Thus, both further investigations of diverse naturalistic stimuli in fMRI, and perhaps the use of more temporally sensitive methods, like MEG (Baillet 2017) or ECoG (Jacobs and Kahana 2010), are likely to inform our understanding of internetwork synchronization patterns and causes.
Aside from the anatomical connectivity and history of coactivation, internetwork synchronization is potentially consistent with a common driver outside of either network: i.e., a brain region or network that both the language and ToM network are synchronized with. Although the deep relationship between language and mentalizing makes a direct-connection account plausible, the existence of potential common drivers cannot be ruled out and deserves further investigation.
One interesting result in the current study emerged in the analysis of LH versus RH language regions’ synchronization with the ToM system. Given the RH language network’s alleged role in nonliteral comprehension and pragmatic reasoning (e.g., Coulson and Williams 2005; Eviatar and Just 2006; Joanette et al. 1990; Kuperberg et al. 2000; Mashal et al. 2005), one might expect stronger correlations between the RH language regions and the ToM network, compared with the LH language regions and the ToM network. Yet we found (in experiments 1b and 3a) that, if anything, ToM regions are more strongly correlated with the LH language fROIs. One thing to keep in mind is that in many prior imaging and patient studies, it is difficult to determine whether the relevant effects arise within the RH language regions or the nearby regions of the ToM network (e.g., the right TPJ, the most selective component of the ToM network; Saxe and Powell 2006). The latter possibility is in line with a few recent neuroimaging studies that have reported responses to nonliteral phenomena like jokes and sarcasm, as well as sensitivity to discourse-level coherence, in ToM regions, and not in the RH homologs of the language regions (e.g., Jacoby and Fedorenko 2018; Kline et al. 2016; Mason et al. 2008; Spotorno et al. 2012).
Pragmatic deficits—which plausibly arise at the interface of language and social cognition—characterize numerous developmental disorders, like autism (e.g., Baron-Cohen 1988, 1997; Dewey and Everard 1974; Happé 1993; Tager-Flusberg 1981a, 1981b) or social communication disorder (e.g., Swineford et al. 2014) and commonly arise in individuals with acquired brain damage (e.g., Boone and Plante 1993; Docking et al. 2000; Harciarek and Jodzio 2005; Joanette et al. 1990; Kaplan et al. 1990; Kertesz et al. 2007; Mcdonald 1992, 1993; McDonald and Pearce 1998; Ozonoff and Miller 1996; Pearce et al. 1998). The current findings might provide a potential neural marker of successful language-ToM system interaction, and future studies could examine potential deviations from the observed pattern in individuals with pragmatic difficulties or in typical development (e.g., Mason et al. 2008). Relatedly, interindividual variability in the strength of the language-ToM correlations among neurotypical adults can in the future be related to behavioral variability in pragmatic abilities.
Finally, as acknowledged earlier in the paper, we here focused on a subset of cognitive processes in each domain. In particular, for language, we focused on higher-level (lexical and combinatorial syntactic/semantic) processes and excluded lower-level perceptual and articulatory motor processes; and for ToM, we focused on the cognitive (representational) rather than affective component of mentalizing. Narrowing the scope in each domain in this way is arguably justifiable because these are the aspects of language and ToM that seem most likely to interact in adult cognition. Indeed, we observed reliable synchronization between the sets of brain regions that support high-level language processing (Fedorenko et al. 2010) and cognitive ToM (Saxe and Kanwisher 2003). However, the design we chose restricts the scope of the conclusions we can draw: namely, we cannot rule out potential additional overlap between the aspects of language and ToM not examined here, or argue that these other aspects do not overlap between the two domains. Indeed, the motivation to attend to other agents and implicit aspects of ToM may be key to language acquisition (e.g., Lohmannet al. 2005; Quinn et al. 2018; Tomasello 1992).
To conclude, we observed significant synchronization of neural fluctuations between the language and ToM networks, during both rest and story comprehension. This synchronization may support successful communication, which requires language and social reasoning to work in concert.
GRANTS
This work was supported by a grant from the Simons Foundation to the Simons Center for the Social Brain at MIT. E. Fedorenko was additionally supported by NIH awards R00-HD057522 and R01-DC016607.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.P., I.A.B., and E.F. conceived and designed research; A.P. and I.A.B. performed experiments; A.P. and I.A.B. analyzed data; A.P., I.A.B., and E.F. interpreted results of experiments; A.P. prepared figures; A.P. and E.F. drafted manuscript; A.P., I.A.B., and E.F. edited and revised manuscript; A.P., I.A.B., and E.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research, MIT. For technical support during scanning, the authors thank Steve Shannon, Atsushi Takahashi, and Sheeba Arnold. We thank Zach Mineroff for help with data collection and organizing the relevant information for this manuscript. We thank Hope Kean for help with figure and manuscript preparation. Finally, we thank other EvLab members for help with data collection.
General
- BOLD
blood oxygenation level-dependent
- ECoG
electrocorticography
- EPI
echo-planar imaging
- Expt.
experiment
- FDR
false discovery rate
- fROI
functional region of interest
- FWHM
full width at half maximum
- GRAPPA
generalized autocalibrating partial parallel acquisition
- LH
left hemisphere
- MD
multiple demand
- MEG
magnetoencephalography
- RH
right hemisphere
- TE
time to echo
- ToM
theory of mind
- TR
time to repeat
Language network
- AngG
angular gyrus
- AntTemp
left anterior temporal cortex
- IFG
inferior frontal gyrus
- IFGorb
IFG orbital portion
- MFG
middle frontal gyrus
- PostTemp
posterior temporal cortex
MD network
- DMPFC
dorsal medial prefrontal cortex
- MMPFC
middle medial prefrontal cortex
- PC
posterior cingulate cortex and precuneus
- TPJ
temporoparietal junction
- VMPFC
ventral medial prefrontal cortex
ToM network
- ACC
anterior cingulate cortex
- IFGop
IFG opercular portion
- MFG
middle frontal gyrus
- MFGorb
orbital portion of MFG
- ParInf
inferior parietal cortex
- ParSup
superior parietal cortex
- PrecG
precentral gyrus
- SMA
supplemental motor area
L and R preceding an abbreviation denote left and right respectively.
REFERENCES
- Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol 60: 693–716, 2009. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apperly IA, Samson D, Carroll N, Hussain S, Humphreys G. Intact first- and second-order false belief reasoning in a patient with severely impaired grammar. Soc Neurosci 1: 334–348, 2006. doi: 10.1080/17470910601038693. [DOI] [PubMed] [Google Scholar]
- Appleton M, Reddy V. Teaching three-year-olds to pass false belief tests: a conversational approach. Soc Dev 5: 275–291, 1996. doi: 10.1111/j.1467-9507.1996.tb00086.x. [DOI] [Google Scholar]
- Asaad WF, Rainer G, Miller EK. Task-specific neural activity in the primate prefrontal cortex. J Neurophysiol 84: 451–459, 2000. doi: 10.1152/jn.2000.84.1.451. [DOI] [PubMed] [Google Scholar]
- Åsberg J. Patterns of language and discourse comprehension skills in school-aged children with autism spectrum disorders. Scand J Psychol 51: 534–539, 2010. doi: 10.1111/j.1467-9450.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- Astington JW, Baird JA, editors. Why Language Matters for Theory of Mind. Oxford, UK: Oxford University Press, 2005. doi: 10.1093/acprof:oso/9780195159912.001.0001 [DOI] [Google Scholar]
- Astington JW, Jenkins JM. A longitudinal study of the relation between language and theory-of-mind development. Dev Psychol 35: 1311–1320, 1999. doi: 10.1037/0012-1649.35.5.1311. [DOI] [PubMed] [Google Scholar]
- Baillet S. Magnetoencephalography for brain electrophysiology and imaging. Nat Neurosci 20: 327–339, 2017. doi: 10.1038/nn.4504. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Social and pragmatic deficits in autism: cognitive or affective? J Autism Dev Disord 18: 379–402, 1988. doi: 10.1007/BF02212194. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: An Essay on Autism and Theory of Mind. Cambridge, MA: MIT Press, 1997. [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition 21: 37–46, 1985. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci USA 108: 7641–7646, 2011. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Lynall ME. Network methods to characterize brain structure and function. In: Cognitive Neurosciences: The Biology of the Mind, edited by Gazzaniga MS, Ivry RB, Mangun GR (5th ed.). New York: Norton, 2013, p. 1–27. [Google Scholar]
- Bassett DS, Wymbs NF, Rombach MP, Porter MA, Mucha PJ, Grafton ST. Task-based core-periphery organization of human brain dynamics. PLOS Comput Biol 9: e1003171, 2013. [Erratum in PLoS Comput Biol 10: e1003617.] doi: 10.1371/journal.pcbi.1003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Dai B. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-27, 2008. http://lme4.r-forge.r-project.org/
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nat Neurosci 6: 448–450, 2003. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Bautista A, Wilson SM. Neural responses to grammatically and lexically degraded speech. Lang Cogn Neurosci 31: 567–574, 2016. doi: 10.1080/23273798.2015.1123281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz A, Jager G, VanRooij R, editors. Game Theory and Pragmatics. Houndmills, UK: Palgrave Macmillan, 2006. doi: 10.1057/9780230285897. [DOI] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci 17: 353–362, 1997. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34: 537–541, 1995. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blank I, Balewski Z, Mahowald K, Fedorenko E. Syntactic processing is distributed across the language system. Neuroimage 127: 307–323, 2016. doi: 10.1016/j.neuroimage.2015.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank I, Kanwisher N, Fedorenko E. A functional dissociation between language and multiple-demand systems revealed in patterns of BOLD signal fluctuations. J Neurophysiol 112: 1105–1118, 2014. doi: 10.1152/jn.00884.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi DE, Wichmann S, Hammarström H, Stadler PF, Christiansen MH. Sound-meaning association biases evidenced across thousands of languages. Proc Natl Acad Sci USA 113: 10818–10823, 2016. doi: 10.1073/pnas.1605782113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone DR, Plante E. Human Communication and Its Disorders. Englewood Cliffs, NJ: Prentice Hall, 1993. [Google Scholar]
- Borrego N. Big cats as a model system for the study of the evolution of intelligence. Behav Processes 141: 261–266, 2017. doi: 10.1016/j.beproc.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Borrego N, Gaines M. Social carnivores outperform asocial carnivores on an innovative problem. Anim Behav 114: 21–26, 2016. doi: 10.1016/j.anbehav.2016.01.013. [DOI] [Google Scholar]
- Braze D, Mencl WE, Tabor W, Pugh KR, Constable RT, Fulbright RK, Magnuson JS, Van Dyke JA, Shankweiler DP. Unification of sentence processing via ear and eye: an fMRI study. Cortex 47: 416–431, 2011. doi: 10.1016/j.cortex.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers L, Ring B. A neuroethological framework for the representation of minds. J Cogn Neurosci 4: 107–118, 1992. doi: 10.1162/jocn.1992.4.2.107. [DOI] [PubMed] [Google Scholar]
- Brown-Schmidt S, Gunlogson C, Tanenhaus MK. Addressees distinguish shared from private information when interpreting questions during interactive conversation. Cognition 107: 1122–1134, 2008. doi: 10.1016/j.cognition.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau EG, Dufour N, Saxe R. Social cognition in members of conflict groups: behavioural and neural responses in Arabs, Israelis and South Americans to each other’s misfortunes. Philos Trans R Soc Lond B Biol Sci 367: 717–730, 2012. doi: 10.1098/rstb.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124: 1–38, 2008. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci 16: 832–837, 2013. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Claxton LJ, Moses LJ. The relation between executive function and theory of mind is more than skin deep. J Cogn Dev 16: 186–197, 2015. doi: 10.1080/15248372.2013.824883. [DOI] [Google Scholar]
- Carlson SM, Moses LJ. Individual differences in inhibitory control and children’s theory of mind. Child Dev 72: 1032–1053, 2001. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- Chai LR, Mattar MG, Blank IA, Fedorenko E, Bassett DS. Functional network dynamics of the language system. Cereb Cortex 26: 4148–4159, 2016. doi: 10.1093/cercor/bhw238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramidaro A, Adenzato M, Enrici I, Erk S, Pia L, Bara BG, Walter H. The intentional network: how the brain reads varieties of intentions. Neuropsychologia 45: 3105–3113, 2007. doi: 10.1016/j.neuropsychologia.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Clark HH, Wilkes-Gibbs D. Referring as a collaborative process. Cognition 22: 1–39, 1986. doi: 10.1016/0010-0277(86)90010-7. [DOI] [PubMed] [Google Scholar]
- Clements WA, Rustin CL, McCallum S. Promoting the transition from implicit to explicit understanding: a training study of false belief. Dev Sci 3: 81–92, 2000. doi: 10.1111/1467-7687.00102. [DOI] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci 4: 8, 2010. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 16: 1348–1355, 2013. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol 22: 1326–1333, 2001. [PMC free article] [PubMed] [Google Scholar]
- Coulson S, Williams RF. Hemispheric asymmetries and joke comprehension. Neuropsychologia 43: 128–141, 2005. doi: 10.1016/j.neuropsychologia.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Dautriche I, Chemla E, Christophe A. Word learning: homophony and the distribution of learning exemplars. Lang Learn Dev 12: 231–251, 2016. doi: 10.1080/15475441.2015.1127163. [DOI] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage 29: 1359–1367, 2006. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- De Villiers J. Language and theory of mind: what are the developmental relationships. In: Understanding Other Minds: Perspectives from Developmental Cognitive Neuroscience (2nd ed.), edited by Baron-Cohen S, Tager-Flusberg H, Cohen DJ. Oxford, UK: Oxford University Press, 2000, p. 83–123. [Google Scholar]
- de Villiers JG, de Villiers PA. The role of language in theory of mind development. Top Lang Disord 34: 313–328, 2014. doi: 10.1097/TLD.0000000000000037. [DOI] [Google Scholar]
- De Villiers PA. The role of language in theory-of-mind development: what deaf children tell us. In: Why Language Matters for Theory of Mind, edited by Astington JW, Baird JA. Oxford, UK: Oxford University Press, 2005, p. 266–298. doi: 10.1093/acprof:oso/9780195159912.003.0013. [DOI] [Google Scholar]
- Deco G, Corbetta M. The dynamical balance of the brain at rest. Neuroscientist 17: 107–123, 2011. doi: 10.1177/1073858409354384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci 12: 43–56, 2011. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Resting brains never rest: computational insights into potential cognitive architectures. Trends Neurosci 36: 268–274, 2013. [Erratum in Trends Neurosci 41: P191, 2018.] doi: 10.1016/j.tins.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Deen B, Koldewyn K, Kanwisher N, Saxe R. Functional organization of social perception and cognition in the superior temporal sulcus. Cereb Cortex 25: 4596–4609, 2015. doi: 10.1093/cercor/bhv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennett DC. Brainstorms. Montgomery, VT: Bradford Books, 1978. [Google Scholar]
- Dennis M, Simic N, Bigler ED, Abildskov T, Agostino A, Taylor HG, Rubin K, Vannatta K, Gerhardt CA, Stancin T, Yeates KO. Cognitive, affective, and conative theory of mind (ToM) in children with traumatic brain injury. Dev Cogn Neurosci 5: 25–39, 2013. doi: 10.1016/j.dcn.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey MA, Everard MP. The near-normal autistic adolescent. J Autism Dev Disord 4: 348–356, 1974. doi: 10.1007/BF02105378. [DOI] [Google Scholar]
- Diaz MT, Hogstrom LJ. The influence of context on hemispheric recruitment during metaphor processing. J Cogn Neurosci 23: 3586–3597, 2011. doi: 10.1162/jocn_a_00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl JJ, Bennetto L, Young EC. Story recall and narrative coherence of high-functioning children with autism spectrum disorders. J Abnorm Child Psychol 34: 83–98, 2006. doi: 10.1007/s10802-005-9003-x. [DOI] [PubMed] [Google Scholar]
- Docking K, Murdoch BE, Jordan FM. Interpretation and comprehension of linguistic humour by adolescents with head injury: a group analysis. Brain Inj 14: 89–108, 2000. doi: 10.1080/026990500120952. [DOI] [PubMed] [Google Scholar]
- Dodell-Feder D, Koster-Hale J, Bedny M, Saxe R. fMRI item analysis in a theory of mind task. Neuroimage 55: 705–712, 2011. doi: 10.1016/j.neuroimage.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Ludy CA, Redfern BB. Pragmatics in the absence of verbal language: descriptions of a severe aphasic and a language-deprived adult. J Neurolinguist 11: 179–190, 1998. doi: 10.1016/S0911-6044(98)00012-8. [DOI] [Google Scholar]
- Dufour N, Redcay E, Young L, Mavros PL, Moran JM, Triantafyllou C, Gabrieli JD, Saxe R. Similar brain activation during false belief tasks in a large sample of adults with and without autism. PLoS One 8: e75468, 2013. doi: 10.1371/journal.pone.0075468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar R. Grooming, Gossip and the Evolution of Language. London: Faber and Faber, 1994. [Google Scholar]
- Dunbar RI, Marriott A, Duncan ND. Human conversational behavior. Hum Nat 8: 231–246, 1997. doi: 10.1007/BF02912493. [DOI] [PubMed] [Google Scholar]
- Dunbar RI, Shultz S. Evolution in the social brain. Science 317: 1344–1347, 2007. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci 14: 172–179, 2010. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23: 475–483, 2000. doi: 10.1016/S0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H. A neural basis for general intelligence. Science 289: 457–460, 2000. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- Dunn J, Brown J, Slomkowski C, Tesla C, Youngblade L. Young children’s understanding of other people’s feelings and beliefs: individual differences and their antecedents. Child Dev 62: 1352–1366, 1991. doi: 10.2307/1130811. [DOI] [PubMed] [Google Scholar]
- Emler N. Gossip, reputation, and social adaptation. In: Good Gossip, edited by Goodman RF, Ben-Ze’ev A. Lawrence, KS: University Press of Kansas, 1994, p. 117–138. [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2: 704–716, 2001. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Eviatar Z, Just MA. Brain correlates of discourse processing: an fMRI investigation of irony and conventional metaphor comprehension. Neuropsychologia 44: 2348–2359, 2006. doi: 10.1016/j.neuropsychologia.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E. The role of domain-general cognitive control in language comprehension. Front Psychol 5: 335, 2014. doi: 10.3389/fpsyg.2014.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Behr MK, Kanwisher N. Functional specificity for high-level linguistic processing in the human brain. Proc Natl Acad Sci USA 108: 16428–16433, 2011. doi: 10.1073/pnas.1112937108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci USA 110: 16616–16621, 2013. doi: 10.1073/pnas.1315235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Hsieh PJ, Nieto-Castañón A, Whitfield-Gabrieli S, Kanwisher N. New method for fMRI investigations of language: defining ROIs functionally in individual subjects. J Neurophysiol 104: 1177–1194, 2010. doi: 10.1152/jn.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Nieto-Castañon A, Kanwisher N. Lexical and syntactic representations in the brain: an fMRI investigation with multi-voxel pattern analyses. Neuropsychologia 50: 499–513, 2012. doi: 10.1016/j.neuropsychologia.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Thompson-Schill SL. Reworking the language network. Trends Cogn Sci 18: 120–126, 2014. doi: 10.1016/j.tics.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Varley R. Language and thought are not the same thing: evidence from neuroimaging and neurological patients. Ann N Y Acad Sci 1369: 132–153, 2016. doi: 10.1111/nyas.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg M, Willer R, Stellar J, Keltner D. The virtues of gossip: reputational information sharing as prosocial behavior. J Pers Soc Psychol 102: 1015–1030, 2012. doi: 10.1037/a0026650. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, Frith CD. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition 57: 109–128, 1995. doi: 10.1016/0010-0277(95)00692-R. [DOI] [PubMed] [Google Scholar]
- Fodor JA. Modularity of Mind: An Essay on Faculty Psychology. Cambridge, MA: MIT Press, 1983. [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711, 2007. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Frank MC, Goodman ND. Predicting pragmatic reasoning in language games. Science 336: 998, 2012. doi: 10.1126/science.1218633. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Yourganov G, Bonilha L, Basilakos A, Den Ouden DB, Rorden C. Revealing the dual streams of speech processing. Proc Natl Acad Sci USA 113: 15108–15113, 2016. doi: 10.1073/pnas.1614038114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. Differential roles for visuospatial and verbal working memory in situation model construction. J Exp Psychol Gen 129: 61–83, 2000. doi: 10.1037/0096-3445.129.1.61. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci 9: 474–480, 2005. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291: 1560–1563, 2001. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Frith U, Happé F. Autism: beyond “theory of mind”. Cognition 50: 115–132, 1994. doi: 10.1016/0010-0277(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Futrell R, Gibson E, Tily H, Blank I, Vishnevetsky A, Piantadosi ST, Fedorenko E. The natural stories corpus (Preprint). arXiv 1708.05763, 2017. [DOI] [PMC free article] [PubMed]
- Futrell R, Mahowald K, Gibson E. Large-scale evidence of dependency length minimization in 37 languages. Proc Natl Acad Sci USA 112: 10336–10341, 2015. [Errata in Proc Natl Acad Sci USA 112: E6407 and E5443–E5444, 2015.] doi: 10.1073/pnas.1502134112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia 38: 11–21, 2000. doi: 10.1016/S0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gibson E, Bergen L, Piantadosi ST. Rational integration of noisy evidence and prior semantic expectations in sentence interpretation. Proc Natl Acad Sci USA 110: 8051–8056, 2013. doi: 10.1073/pnas.1216438110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: a comparison of theory of mind tasks. J Cogn Neurosci 19: 1803–1814, 2007. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- Gohel SR, Biswal BB. Functional integration between brain regions at rest occurs in multiple-frequency bands. Brain Connect 5: 23–34, 2015. doi: 10.1089/brain.2013.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AE. Constructions: A Construction Grammar Approach to Argument Structure. Chicago, IL: University of Chicago Press, 1995. [Google Scholar]
- Gómez S, Jensen P, Arenas A. Analysis of community structure in networks of correlated data. Phys Rev E Stat Nonlin Soft Matter Phys 80: 016114, 2009. doi: 10.1103/PhysRevE.80.016114. [DOI] [PubMed] [Google Scholar]
- Goodman ND, Frank MC. Pragmatic language interpretation as probabilistic inference. Trends Cogn Sci 20: 818–829, 2016. doi: 10.1016/j.tics.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Goodman ND, Stuhlmüller A. Knowledge and implicature: modeling language understanding as social cognition. Top Cogn Sci 5: 173–184, 2013. doi: 10.1111/tops.12007. [DOI] [PubMed] [Google Scholar]
- Gratton C, Laumann TO, Nielsen AN, Greene DJ, Gordon EM, Gilmore AW, Nelson SM, Coalson RS, Snyder AZ, Schlaggar BL, Dosenbach NUF, Petersen SE. Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation. Neuron 98: 439–452.e5, 2018. doi: 10.1016/j.neuron.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA 86: 1698–1702, 1989. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258, 2003. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice HP. Meaning. Philos Rev 66: 377–388, 1957. doi: 10.2307/2182440. [DOI] [Google Scholar]
- Grice HP. Utterer’s meaning, sentence-meaning, and word-meaning. Found Lang 4: 225–242, 1968. [Google Scholar]
- Grice HP. Logic and conversation. In: Syntax and Semantics: Speech Acts, edited by Cole P, Morgan J. New York: Academic, 1975, vol. 3, p. 41–58. [Google Scholar]
- Grosbras MH, Beaton S, Eickhoff SB. Brain regions involved in human movement perception: a quantitative voxel-based meta-analysis. Hum Brain Mapp 33: 431–454, 2012. doi: 10.1002/hbm.21222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CM, Tager-Flusberg H. The influence of language on theory of mind: a training study. Dev Sci 6: 346–359, 2003. doi: 10.1111/1467-7687.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé FG. Communicative competence and theory of mind in autism: a test of relevance theory. Cognition 48: 101–119, 1993. doi: 10.1016/0010-0277(93)90026-R. [DOI] [PubMed] [Google Scholar]
- Harciarek M, Jodzio K. Neuropsychological differences between frontotemporal dementia and Alzheimer’s disease: a review. Neuropsychol Rev 15: 131–145, 2005. doi: 10.1007/s11065-005-7093-4. [DOI] [PubMed] [Google Scholar]
- Hartigan JA. Clustering Algorithms. New York: Wiley, 1975. [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc Natl Acad Sci USA 105: 16039–16044, 2008. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Curr Opin Neurobiol 18: 153–158, 2008. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Heller D, Grodner D, Tanenhaus MK. The role of perspective in identifying domains of reference. Cognition 108: 831–836, 2008. doi: 10.1016/j.cognition.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Heyes CM, Frith CD. The cultural evolution of mind reading. Science 344: 1243091, 2014. doi: 10.1126/science.1243091. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Raichle ME, Mitra A, Specht K. On the existence of a generalized non-specific task-dependent network. Front Hum Neurosci 9: 430, 2015. doi: 10.3389/fnhum.2015.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurford JR, Studdert-Kennedy M, Knight C. Approaches to the Evolution of Language: Social and Cognitive Bases. Cambridge, UK: Cambridge University Press, 1998. [Google Scholar]
- Jackendoff R. Foundation of Language: Brain, Meaning, Grammar, Evolution. New York: Oxford University Press, 2002. doi: 10.1093/acprof:oso/9780198270126.001.0001 [DOI] [Google Scholar]
- Jacobs J, Kahana MJ. Direct brain recordings fuel advances in cognitive electrophysiology. Trends Cogn Sci 14: 162–171, 2010. doi: 10.1016/j.tics.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby N, Bruneau E, Koster-Hale J, Saxe R. Localizing Pain Matrix and Theory of Mind networks with both verbal and non-verbal stimuli. Neuroimage 126: 39–48, 2016. doi: 10.1016/j.neuroimage.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby N, Fedorenko E. Discourse-level comprehension engages medial frontal Theory of Mind brain regions even for expository texts. Lang Cogn Neurosci 1–17, 2018. 10.1080/23273798.2018.1525494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobson R. Six Lectures on Sound and Meaning. Hassocks, UK: Harvester, 1978, p. 19. [Google Scholar]
- Janke V, Perovic A. Intact grammar in HFA? Evidence from control and binding. Lingua 164: 68–86, 2015. doi: 10.1016/j.lingua.2015.06.009. [DOI] [Google Scholar]
- Joanette Y, Goulet P, Hannequin D, Boeglin J. Right Hemisphere and Verbal Communication. New York: Springer, 1990. [Google Scholar]
- Jouravlev O, Zheng D, Balewski Z, Goldin-Meadow S, Fedorenko E. Gesture processing does not engage high-level language processing brain regions (Preprint). OSF, November 9, 2018. osf.io/zjpgf.
- Julian JB, Fedorenko E, Webster J, Kanwisher N. An algorithmic method for functionally defining regions of interest in the ventral visual pathway. Neuroimage 60: 2357–2364, 2012. doi: 10.1016/j.neuroimage.2012.02.055. [DOI] [PubMed] [Google Scholar]
- Kaplan JA, Brownell HH, Jacobs JR, Gardner H. The effects of right hemisphere damage on the pragmatic interpretation of conversational remarks. Brain Lang 38: 315–333, 1990. doi: 10.1016/0093-934X(90)90117-Y. [DOI] [PubMed] [Google Scholar]
- Keller CJ, Bickel S, Entz L, Ulbert I, Milham MP, Kelly C, Mehta AD. Intrinsic functional architecture predicts electrically evoked responses in the human brain. Proc Natl Acad Sci USA 108: 10308–10313, 2011. [Erratum in Proc Natl Acad Sci USA 108: 17234, 2011.] doi: 10.1073/pnas.1019750108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, Blair M, McMonagle P, Munoz DG. The diagnosis and course of frontotemporal dementia. Alzheimer Dis Assoc Disord 21: 155–163, 2007. doi: 10.1097/WAD.0b013e31806547eb. [DOI] [PubMed] [Google Scholar]
- Kirby S. Syntax without natural selection: how compositionality emerges from vocabulary in a population of learners. In: The Evolutionary Emergence of Language: Social Function and the Origins of Linguistic Form, edited by Knight C. Cambridge, UK: Cambridge University Press, 2000, p. 303–323. doi: 10.1017/CBO9780511606441.019. [DOI] [Google Scholar]
- Kline M, Gallée J, Balewski Z, Fedorenko E. The role of the Theory of Mind network in understanding jokes (Preprint). PsyArXiv 2016. doi: 10.31234/osf.io/h2nyx. [DOI]
- Koster-Hale J, Saxe R. Functional neuroimaging of theory of mind. In: Understanding Other Minds: Perspectives from Developmental Social Neuroscience (3rd ed.), edited by Baron-Cohen S, Tager-Flusberg H, Lombardo MV. Oxford, UK: Oxford University Press, 2013, p. 132–163. doi: 10.1093/acprof:oso/9780199692972.003.0009. [DOI] [Google Scholar]
- Kuperberg GR, McGuire PK, Bullmore ET, Brammer MJ, Rabe-Hesketh S, Wright IC, Lythgoe DJ, Williams SC, David AS. Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: an fMRI study. J Cogn Neurosci 12: 321–341, 2000. doi: 10.1162/089892900562138. [DOI] [PubMed] [Google Scholar]
- Ladefoged P, Maddieson I. The Sounds of the World’s Languages. Oxford, UK: Blackwell, 1996. [Google Scholar]
- Lerner Y, Honey CJ, Silbert LJ, Hasson U. Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J Neurosci 31: 2906–2915, 2011. doi: 10.1523/JNEUROSCI.3684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson SC. Turn-taking in human communication—origins and implications for language processing. Trends Cogn Sci 20: 6–14, 2016. doi: 10.1016/j.tics.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Lewis C, Osborne A. Three-year-olds’ problems with false belief: conceptual deficit or linguistic artifact? Child Dev 61: 1514–1519, 1990. doi: 10.2307/1130760. [DOI] [PubMed] [Google Scholar]
- Lihoreau M, Latty T, Chittka L. An exploration of the social brain hypothesis in insects. Front Physiol 3: 442, 2012. doi: 10.3389/fphys.2012.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann H, Tomasello M. The role of language in the development of false belief understanding: a training study. Child Dev 74: 1130–1144, 2003. doi: 10.1111/1467-8624.00597. [DOI] [PubMed] [Google Scholar]
- Lohmann H, Tomasello M, Meyer S. Linguistic Communication and Social Understanding. Oxford, UK: Oxford University Press, 2005, p. 245–265. [Google Scholar]
- Lord C, Paul R. Language and communication in autism. In: Handbook of Autism and Pervasive Developmental Disorders (2nd ed.), edited by Cohen D, Volkmar F. NewYork: Wiley, 1997, p. 195–225. [Google Scholar]
- Lynch LK, Lu KH, Wen H, Zhang Y, Saykin AJ, Liu Z. Task-evoked functional connectivity does not explain functional connectivity differences between rest and task conditions. Hum Brain Mapp 39: 4939–4948, 2018. doi: 10.1002/hbm.24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddieson I, Disner SF. Patterns of Sounds. Cambridge, UK: Cambridge University Press, 1984. doi: 10.1017/CBO9780511753459. [DOI] [Google Scholar]
- Mahowald K, Fedorenko E. Reliable individual-level neural markers of high-level language processing: A necessary precursor for relating neural variability to behavioral and genetic variability. Neuroimage 139: 74–93, 2016. doi: 10.1016/j.neuroimage.2016.05.073. [DOI] [PubMed] [Google Scholar]
- Malle BF. The relation between language and theory of mind in development and evolution. In: The Evolution of Language out of Pre-Language, edited by Givón T, Malle BF. Amsterdam: Benjamins, 2002, p. 265–284. doi: 10.1075/tsl.53.14mal. [DOI] [Google Scholar]
- Mar RA. The neural bases of social cognition and story comprehension. Annu Rev Psychol 62: 103–134, 2011. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T. The role of the right hemisphere in processing nonsalient metaphorical meanings: application of principal components analysis to fMRI data. Neuropsychologia 43: 2084–2100, 2005. doi: 10.1016/j.neuropsychologia.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia 46: 269–280, 2008. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo C, Knutsen PM, Tsai PS, Shih AY, Kleinfeld D. Entrainment of arteriole vasomotor fluctuations by neural activity is a basis of blood-oxygenation-level-dependent “resting-state” connectivity. Neuron 96: 936–948.e3, 2017. doi: 10.1016/j.neuron.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Tamura K, Koyano KW, Takeuchi D, Adachi Y, Osada T, Miyashita Y. Direct comparison of spontaneous functional connectivity and effective connectivity measured by intracortical microstimulation: an fMRI study in macaque monkeys. Cereb Cortex 21: 2348–2356, 2011. doi: 10.1093/cercor/bhr019. [DOI] [PubMed] [Google Scholar]
- Mcdonald S. Communication disorders following closed head injury: new approaches to assessment and rehabilitation. Brain Inj 6: 283–292, 1992. doi: 10.3109/02699059209029670. [DOI] [PubMed] [Google Scholar]
- Mcdonald S. Pragmatic language skills after closed head injury: ability to meet the informational needs of the listener. Brain Lang 44: 28–46, 1993. doi: 10.1006/brln.1993.1003. [DOI] [PubMed] [Google Scholar]
- McDonald S, Pearce S. Requests that overcome listener reluctance: impairment associated with executive dysfunction in brain injury. Brain Lang 61: 88–104, 1998. doi: 10.1006/brln.1997.1846. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Thompson CK, Weintraub S, Rogalski EJ. The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain 138: 2423–2437, 2015. doi: 10.1093/brain/awv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan K, Astington JW, Dack LA. Language and theory of mind: meta-analysis of the relation between language ability and false-belief understanding. Child Dev 78: 622–646, 2007. doi: 10.1111/j.1467-8624.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- Mineroff Z, Blank IA, Mahowald K, Fedorenko E. A robust dissociation among the language, multiple demand, and default mode networks: evidence from inter-region correlations in effect size. Neuropsychologia 119: 501–511, 2018. doi: 10.1016/j.neuropsychologia.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Chen Q, Zhang Y, Wang Z, Faseyitan OK, Coslett HB, Schwartz MF. Neural organization of spoken language revealed by lesion-symptom mapping. Nat Commun 6: 6762, 2015. doi: 10.1038/ncomms7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DJ, Bell AH, Buckley MJ, Mitchell AS, Sallet J, Duncan J. A putative multiple-demand system in the macaque brain. J Neurosci 36: 8574–8585, 2016. doi: 10.1523/JNEUROSCI.0810-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Rettinger DA, Shah P, Hegarty M. How are visuospatial working memory, executive functioning, and spatial abilities related? A latent-variable analysis. J Exp Psychol Gen 130: 621–640, 2001. doi: 10.1037/0096-3445.130.4.621. [DOI] [PubMed] [Google Scholar]
- Monti MM, Parsons LM, Osherson DN. Thought beyond language: neural dissociation of algebra and natural language. Psychol Sci 23: 914–922, 2012. doi: 10.1177/0956797612437427. [DOI] [PubMed] [Google Scholar]
- Nadig AS, Sedivy JC. Evidence of perspective-taking constraints in children’s on-line reference resolution. Psychol Sci 13: 329–336, 2002. doi: 10.1111/j.0956-7976.2002.00460.x. [DOI] [PubMed] [Google Scholar]
- Newman ME, Girvan M. Finding and evaluating community structure in networks. Phys Rev E Stat Nonlin Soft Matter Phys 69: 026113, 2004. doi: 10.1103/PhysRevE.69.026113. [DOI] [PubMed] [Google Scholar]
- Nieto-Castañón A, Fedorenko E. Subject-specific functional localizers increase sensitivity and functional resolution of multi-subject analyses. Neuroimage 63: 1646–1669, 2012. doi: 10.1016/j.neuroimage.2012.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman-Haignere S, Kanwisher NG, McDermott JH. Distinct cortical pathways for music and speech revealed by hypothesis-free voxel decomposition. Neuron 88: 1281–1296, 2015. doi: 10.1016/j.neuron.2015.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA, Krakauer DC. The evolution of language. Proc Natl Acad Sci USA 96: 8028–8033, 1999. doi: 10.1073/pnas.96.14.8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Miller JN. An exploration of right-hemisphere contributions to the pragmatic impairments of autism. Brain Lang 52: 411–434, 1996. doi: 10.1006/brln.1996.0022. [DOI] [PubMed] [Google Scholar]
- Pearce S, McDonald S, Coltheart M. Interpreting ambiguous advertisements: the effect of frontal lobe damage. Brain Cogn 38: 150–164, 1998. doi: 10.1006/brcg.1998.1018. [DOI] [PubMed] [Google Scholar]
- Penfield W, Roberts L. Speech and Brain Mechanisms. Princeton, NJ: Princeton University Press, 1959. [Google Scholar]
- Peterson CC, Siegal M. Insights into theory of mind from deafness and autism. Mind Lang 15: 123–145, 2000. doi: 10.1111/1468-0017.00126. [DOI] [Google Scholar]
- Piantadosi ST, Tily H, Gibson E. Word lengths are optimized for efficient communication. Proc Natl Acad Sci USA 108: 3526–3529, 2011. doi: 10.1073/pnas.1012551108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinker S. The cognitive niche: coevolution of intelligence, sociality, and language. Proc Natl Acad Sci USA 107, Suppl 2: 8993–8999, 2010. doi: 10.1073/pnas.0914630107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, Nichols TE, Poline JB, Vul E, Yarkoni T. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 18: 115–126, 2017. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LJ, Carey S. Executive function depletion in children and its impact on theory of mind. Cognition 164: 150–162, 2017. doi: 10.1016/j.cognition.2017.03.022. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron 72: 665–678, 2011. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav Brain Sci 1: 515–526, 1978. doi: 10.1017/S0140525X00076512. [DOI] [Google Scholar]
- Pritchett BL, Hoeflin C, Koldewyn K, Dechter E, Fedorenko E. High-level language processing regions are not engaged in action observation or imitation. J Neurophysiol 120: 2555–2570, 2018. doi: 10.1152/jn.00222.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyers JE, Senghas A. Language promotes false-belief understanding: evidence from learners of a new sign language. Psychol Sci 20: 805–812, 2009. doi: 10.1111/j.1467-9280.2009.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn S, Donnelly S, Kidd E. The relationship between symbolic play and language acquisition: a meta-analytic review. Dev Rev 49: 121–135, 2018. doi: 10.1016/j.dr.2018.05.005. [DOI] [Google Scholar]
- Reader SM, Laland KN. Social intelligence, innovation, and enhanced brain size in primates. Proc Natl Acad Sci USA 99: 4436–4441, 2002. doi: 10.1073/pnas.062041299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H, Koster-Hale J, Caselli NK, Magid RW, Benedict R, Olson H, Pyers J, Saxe R. How language facilitates theory of mind development: behavioral and FMRI evidence from individuals with delayed access to language (Preprint). PsyArXiv, 2018. doi: 10.31234/osf.io/8krmb. [DOI]
- Roth D, Leslie AM. Solving belief problems: toward a task analysis. Cognition 66: 1–31, 1998. doi: 10.1016/S0010-0277(98)00005-5. [DOI] [PubMed] [Google Scholar]
- Rubio-Fernández P, Geurts B. How to pass the false-belief task before your fourth birthday. Psychol Sci 24: 27–33, 2013. doi: 10.1177/0956797612447819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Fernández P, Geurts B. Don’t mention the marble! The role of attentional processes in false-belief tasks. Rev Phil Psychol 7: 835–850, 2016. doi: 10.1007/s13164-015-0290-z. [DOI] [Google Scholar]
- Ruby P, Decety J. What you believe versus what you think they believe: a neuroimaging study of conceptual perspective-taking. Eur J Neurosci 17: 2475–2480, 2003. doi: 10.1046/j.1460-9568.2003.02673.x. [DOI] [PubMed] [Google Scholar]
- Saxe R, Brett M, Kanwisher N. Divide and conquer: a defense of functional localizers. Neuroimage 30: 1088–1096, 2006a. doi: 10.1016/j.neuroimage.2005.12.062. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Curr Opin Neurobiol 16: 235–239, 2006. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage 19: 1835–1842, 2003. doi: 10.1016/S1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol Sci 17: 692–699, 2006. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Saxe R, Schulz LE, Jiang YV. Reading minds versus following rules: dissociating theory of mind and executive control in the brain. Soc Neurosci 1: 284–298, 2006b. doi: 10.1080/17470910601000446. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia 43: 1391–1399, 2005. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Saxe R, Young L. Theory of mind: how brains think about thoughts. In: The Handbook of Cognitive Neuroscience, edited by Ochsner K, Kosslyn SM. Oxford, UK: Oxford University Press, 2016. [Google Scholar]
- Schick B, de Villiers J, de Villiers P, Hoffmeister B. Theory of mind: language and cognition in deaf children. ASHA Lead 7: 6–14, 2002. doi: 10.1044/leader.FTR1.07222002.6. [DOI] [Google Scholar]
- Schick B, de Villiers P, de Villiers J, Hoffmeister R. Language and theory of mind: a study of deaf children. Child Dev 78: 376–396, 2007. doi: 10.1111/j.1467-8624.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- Schölvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci USA 107: 10238–10243, 2010. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TL, Gallée J, Fedorenko E. A new fun and robust version of an fMRI localizer for the frontotemporal language system. Cogn Neurosci 8: 167–176, 2016. doi: 10.1080/17588928.2016.1201466. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 132: 617–627, 2009. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp 29: 751–761, 2008. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver NC, Dunlap WP. Averaging correlation coefficients: should Fisher’s z transformation be used? J Appl Psychol 72: 146–148, 1987. doi: 10.1037/0021-9010.72.1.146. [DOI] [Google Scholar]
- Singer T, Lamm C. The social neuroscience of empathy. Ann N Y Acad Sci 1156: 81–96, 2009. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Slaughter V, Gopnik A. Conceptual coherence in the child’s theory of mind: training children to understand belief. Child Dev 67: 2967–2988, 1996. doi: 10.2307/1131762. [DOI] [PubMed] [Google Scholar]
- Smith K, Brighton H, Kirby S. Complex systems in language evolution: the cultural emergence of compositional structure. Adv Complex Syst 6: 537–558, 2003. doi: 10.1142/S0219525903001055. [DOI] [Google Scholar]
- Southgate V, Chevallier C, Csibra G. Seventeen-month-olds appeal to false beliefs to interpret others’ referential communication. Dev Sci 13: 907–912, 2010. doi: 10.1111/j.1467-7687.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- Sperber D, Wilson D. Relevance: Communication and cognition. Cambridge, MA: Harvard University Press, 1986, vol 142. [Google Scholar]
- Sperber D, Wilson D. Pragmatics, modularity and mind-reading. Mind Lang 17: 3–23, 2002. doi: 10.1111/1468-0017.00186. [DOI] [Google Scholar]
- Spotorno N, Koun E, Prado J, Van Der Henst JB, Noveck IA. Neural evidence that utterance-processing entails mentalizing: the case of irony. Neuroimage 63: 25–39, 2012. doi: 10.1016/j.neuroimage.2012.06.046. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci 21: 489–510, 2009. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Swineford LB, Thurm A, Baird G, Wetherby AM, Swedo S. Social (pragmatic) communication disorder: a research review of this new DSM-5 diagnostic category. J Neurodev Disord 6: 41, 2014. doi: 10.1186/1866-1955-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H. On the nature of linguistic functioning in early infantile autism. J Autism Dev Disord 11: 45–56, 1981a. doi: 10.1007/BF01531340. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. Sentence comprehension in autistic children. Appl Psycholinguist 2: 5–24, 1981b. doi: 10.1017/S014271640000062X. [DOI] [Google Scholar]
- Tager-Flusberg H. Defining language phenotypes in autism. Clin Neurosci Res 6: 219–224, 2006. doi: 10.1016/j.cnr.2006.06.007. [DOI] [Google Scholar]
- Tager-Flusberg H, Paul R, Lord C. Language and communication in autism. In: Handbook of Autism and Pervasive Developmental Disorders: Diagnosis, Development, Neurobiology, and Behavior (3rd ed.), edited by Volkmar F, Paul R, Klin A, Cohen D. Hoboken, NJ: Wiley, 2005, vol. 1, p. 335–364. [Google Scholar]
- Tavor I, Parker Jones O, Mars RB, Smith SM, Behrens TE, Jbabdi S. Task-free MRI predicts individual differences in brain activity during task performance. Science 352: 216–220, 2016. doi: 10.1126/science.aad8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzi A, Marinis T, Francis K. The interface of syntax with pragmatics and prosody in children with Autism Spectrum Disorders. J Autism Dev Disord 46: 2692–2706, 2016. doi: 10.1007/s10803-016-2811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler J, Eubank S, Longtin A, Galdrikian B, Farmer JD. Testing for nonlinearity in time series: the method of surrogate data. Physica D 58: 77–94, 1992. doi: 10.1016/0167-2789(92)90102-S. [DOI] [Google Scholar]
- Tomasello M. The social bases of language acquisition. Soc Dev 1: 67–87, 1992. doi: 10.1111/j.1467-9507.1992.tb00135.x. [DOI] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15: 273–289, 2002. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vagharchakian L, Dehaene-Lambertz G, Pallier C, Dehaene S. A temporal bottleneck in the language comprehension network. J Neurosci 32: 9089–9102, 2012. doi: 10.1523/JNEUROSCI.5685-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varley R, Siegal M. Evidence for cognition without grammar from causal reasoning and ‘theory of mind’ in an agrammatic aphasic patient. Curr Biol 10: 723–726, 2000. doi: 10.1016/S0960-9822(00)00538-8. [DOI] [PubMed] [Google Scholar]
- Varley R, Siegal M, Want SC. Severe impairment in grammar does not preclude theory of mind. Neurocase 7: 489–493, 2001. doi: 10.1093/neucas/7.6.489. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happé F, Falkai P, Maier W, Shah NJ, Fink GR, Zilles K. Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage 14: 170–181, 2001. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- von dem Hagen EA, Stoyanova RS, Baron-Cohen S, Calder AJ. Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Soc Cogn Affect Neurosci 8: 694–701, 2013. doi: 10.1093/scan/nss053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature 411: 953–956, 2001. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- Warren DE, Sutterer MJ, Bruss J, Abel TJ, Jones A, Kawasaki H, Voss M, Cassell M, Howard MA, Tranel D. Surgically disconnected temporal pole exhibits resting functional connectivity with remote brain regions. bioRxiv 127571, 2017. doi: 10.1101/127571. [DOI]
- Watson AC, Painter KM, Bornstein MH. Longitudinal relations between 2-year-olds’ language and 4-year-olds’ theory of mind. J Cogn Dev 2: 449–457, 2001. doi: 10.1207/S15327647JCD0204_5. [DOI] [Google Scholar]
- Wellman H. The Child’s Theory of Mind: The Development of Conscious Cognition. San Diego, CA: Academic, 1985. [Google Scholar]
- Wellman HM. A child’s theory of mind. The Growth of Insight in the Child, Madison, WI, 1979. [Google Scholar]
- Wellman HM, Cross D, Watson J. Meta-analysis of theory-of-mind development: the truth about false belief. Child Dev 72: 655–684, 2001. doi: 10.1111/1467-8624.00304. [DOI] [PubMed] [Google Scholar]
- Whitaker HA, Ojemann GA. Graded localisation of naming from electrical stimulation mapping of left cerebral cortex. Nature 270: 50–51, 1977. doi: 10.1038/270050a0. [DOI] [PubMed] [Google Scholar]
- Whitlow CT, Casanova R, Maldjian JA. Effect of resting-state functional MR imaging duration on stability of graph theory metrics of brain network connectivity. Radiology 259: 516–524, 2011. doi: 10.1148/radiol.11101708. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castañon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2: 125–141, 2012. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wilkinson KM. Profiles of language and communication skills in autism. Ment Retard Dev Disabil Res Rev 4: 73–79, 1998. doi:. [DOI] [Google Scholar]
- Willems RM, Benn Y, Hagoort P, Toni I, Varley R. Communicating without a functioning language system: implications for the role of language in mentalizing. Neuropsychologia 49: 3130–3135, 2011. doi: 10.1016/j.neuropsychologia.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Wimmer H, Perner J. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition 13: 103–128, 1983. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Woensdregt M, Smith K. Pragmatics and language evolution. In: Oxford Research Encyclopedia of Linguistics, edited by Aronoff M. New York: Oxford University Press, 2017. doi: 10.1093/acrefore/9780199384655.013.321. [DOI] [Google Scholar]
- Woolfe T, Want SC, Siegal M. Signposts to development: theory of mind in deaf children. Child Dev 73: 768–778, 2002. doi: 10.1111/1467-8624.00437. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106: 1125–1165, 2011. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Dodell-Feder D, Saxe R. What gets the attention of the temporo-parietal junction? An fMRI investigation of attention and theory of mind. Neuropsychologia 48: 2658–2664, 2010. doi: 10.1016/j.neuropsychologia.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Zipf GK. Human Behaviour and the Principle of Least Effort. Cambridge, MA: Addison-Wesley, 1949. [Google Scholar]