Abstract
Moderate evidence exists regarding percutaneous epidural adhesiolysis (PEA) being an effective treatment for lumbar spinal stenosis (LSS). Although PEA is successfully performed using balloon-less epidural catheters, many patients with severe adhesions cannot obtain satisfactory results. Combined treatment with balloon-inflatable catheters for PEA and balloon decompression recently demonstrated sufficient pain relief and functional improvement in patients with intractable LSS. We compared the effects of PEA and balloon decompression in patients with intractable LSS who did not undergo PEA and those who were unresponsive to previous PEA with a balloon-less catheter.
We examined 315 patients who underwent PEA and balloon decompression with balloon-inflatable catheters. Patients with intractable LSS were divided into those without previous PEA (No-PEA) and those unresponsive to previous PEA using balloon-less catheters (Prev-PEA). The numeric rating scale, Oswestry disability index, and global perceived effect of satisfaction scale were measured at 0, 1, 3, and 6 months after the intervention. Responder analysis was performed based on changes in measured scales and indices.
A successful treatment response was observed at 1, 3, and 6 months after the intervention in 56.4%, 42.7%, and 32.9%, respectively, of the No-PEA group and in 48.9%, 37.8%, and 25.6%, respectively, of the Prev-PEA group. No significant between-group differences were detected. Pain intensities and functional status improved and were maintained throughout follow-up after PEA with balloon decompression using balloon-inflatable catheters.
This modality may represent a useful alternative to overcome the limitations of preexisting adhesiolysis procedures.
Keywords: balloon decompression, chronic pain, epidural adhesiolysis, lumbar, radiculopathy, spinal stenosis
1. Introduction
In the elderly, degenerative changes of the lumbar spine are one of the most common causes of lumbar spinal stenosis (LSS).[1] Although surgical treatment for LSS for leg pain relief and maintaining back-related functions is favored,[2] nonsurgical treatment for the initial management of LSS has been recommended.[3] Nonsurgical treatment for LSS generally includes exercise, physical therapy, medical treatment, and conventional interventional procedures such as epidural steroid injection (ESI).[3] In addition, there is moderate evidence that percutaneous epidural adhesiolysis (PEA) is more effective than conventional ESI for treating spinal stenosis and lumbar radiculopathy.[4,5] Therefore, PEA has been recently recommended over surgical treatments for patients who fail to respond to conventional ESI.[6,7]
PEA is usually performed with a Racz catheter or a steerable epidural catheter such as the NaviCath (Myelotec, Roswell, GA).[7,8] However, the long-term effects of this method remain uncertain and controversial. Because the correct placement of these catheters is difficult to achieve in patients with severe adhesions, many patients with severe adhesions do not obtain satisfactory results after PEA.[9,10] Choi et al recently demonstrated the efficacy of PEA and balloon decompression with a newly developed inflatable balloon catheter (ZiNeu; JUVENUI, Seoul, Korea) in patients with intractable LSS.[11,12] Distension of the epidural space by intermittent ballooning can lead to more effective mechanical detachment of a perineural adhesion and achieve more efficient delivery of epidural injections to the target region (s) by increasing the marginal space of the stenotic area.[12]
Therefore, we compared the effects of combined PEA with balloon decompression in patients with intractable LSS who did not undergo PEA and those who were unresponsive to previous PEA using a balloon-less catheter.
2. Methods
This retrospective study was conducted at the pain management clinic at Asan Medical Center, Seoul, Republic of Korea. The necessity for obtaining informed consent was waived as only recorded data were reviewed. We reviewed patient electronic medical records for all necessary data that were itemized and recorded at their visit to the pain clinic. This retrospective study was approved by the institutional review board of Asan Medical Center (approval number, 2015-1201).
2.1. Participants
This retrospective study included patients who underwent PEA and balloon decompression with a ZiNeu catheter between January and December 2014. Inclusion criteria were as follows:
-
(1)
chronic (at least 3 months) LSS in patients aged ≥40 years with dominant radicular leg pain, less severe back pain, and neurogenic intermittent claudication;
-
(2)
intensity score of ≥6/10 on the numerical rating scale (NRS);
-
(3)
confirmed diagnosis of spinal stenosis with its type and grade by magnetic resonance imaging (MRI);
-
(4)
previous failure of conservative managements such as exercise therapy, physical therapy, or analgesic medications; and
-
(5)
no long-term (i.e., <1 month) effect (minimal pain reduction response, <50%) of conventional interventional procedures, such as caudal, interlaminar, or transforaminal ESI, or even PEA using a balloon-less catheter (Racz or NaviCath).
Exclusion criteria were as follows:
-
(1)
age of <40 years,
-
(2)
acute pain for <3 months,
-
(3)
could not exclude a confounding diagnosis of vascular disease or a disease of other origins,
-
(4)
signs of progressive neurological deficits or motor weakness,
-
(5)
allergies to steroids or contrast dyes,
-
(6)
coagulopathy,
-
(7)
uncontrollable or unstable opioid use,
-
(8)
pregnancy or lactation,
-
(9)
systemic or injection site infection,
-
(10)
malignancy,
-
(11)
unstable medical or psychiatric condition, and
-
(12)
a history of prior lumbar spine surgery.
2.2. Intervention technique: percutaneous epidural adhesiolysis and decompression using an inflatable balloon catheter
In this study, all procedures were performed on an outpatient basis, and no premedication or sedatives were used. Fluoroscopic guidance was implemented in all cases. A single fluoroscopy C-arm system (OEC 9800; General Electric Healthcare, Little Chalfont, United Kingdom) was used. Each patient was placed in the prone position with a pillow under the abdomen to minimize lumbar lordosis. After sterile preparation for the procedure, the skin and soft tissues were infiltrated with 1% lidocaine. A 10-G guide needle, which was custom designed to prevent cutting or skiving of the catheter, was inserted into the epidural space through the sacral hiatus under intermittent fluoroscopy. The epidural space was identified based on injecting approximately 8 mL diluted contrast medium (Omnipaque; Nycomed Imaging AS, Oslo, Norway) under fluoroscopy. The diluted contrast mixture comprised approximately 4 mL pure contrast medium, 4 mL 1% lidocaine, and 1500 IU hyaluronidase. Filling defects were identified by examining the contrast flow. If intravascular placement of the needle or contrast occurred, the needle was removed and repositioned.
After appropriately identifying the epidurogram and target areas, a ZiNeu catheter was advanced through the guide needle to the area of the filling defect or to the pathology site, as determined by MRI or symptomatology. Gentle mechanical adhesiolysis and decompression with a ZiNeu catheter were performed at the appropriate target sites (i.e., the central ventral and dorsal epidural spaces, lateral recess area, and/or each intervertebral foramen). Epidural decompression and adhesiolysis were performed using a gentle side-to-side movement of the catheter with intermittent ballooning (Fig. 1). The balloon was then filled with 0.13 mL contrast agent using a 1-mL Luer-Lock syringe (BD Medical, Franklin Lakes, NJ), and each ballooning process was limited to 5 seconds.[13] The extent of balloon inflation was adjusted on the basis of the degree of pain; if moderate-to-severe pain was noted during balloon inflation, no further attempt was made because of safety concerns. The catheter only moved in the deflated state. After adhesiolysis and decompression, 1 mL pure contrast medium was injected to detect subarachnoid or intravascular filling and to ensure satisfactory filling of previous defects. Then, 2 mL 1% lidocaine with steroid (5 mg dexamethasone) was injected at each target site. At the end of the procedure, a Perifix epidural catheter (B. Braun Melsungen AG, Melsungen, Germany) was left at the main target site through the ZiNeu catheter lumen. After confirming the position of the Perifix catheter tip, the ZiNeu catheter was removed. In the recovery room, a test injection of 2 mL lidocaine was administered via the Perifix catheter. After 10– 15 min of monitoring, another 4 mL 10% hypertonic saline was injected via the Perifix catheter. The Perifix catheter was maintained in place for a 2-day drug injection regimen. The catheter was removed on day 2 post-procedure after the same drugs (10–15 minutes after a test injection of 2 mL 1% lidocaine and 4 mL of 10% hypertonic saline with 5 mg dexamethasone) were injected again. All patients returned home and visited the outpatient clinic on the second day. After confirming that there were no complications such as infection, the drugs were administered on the second day. The patients were discharged after confirmation that there were no complications.
Figure 1.
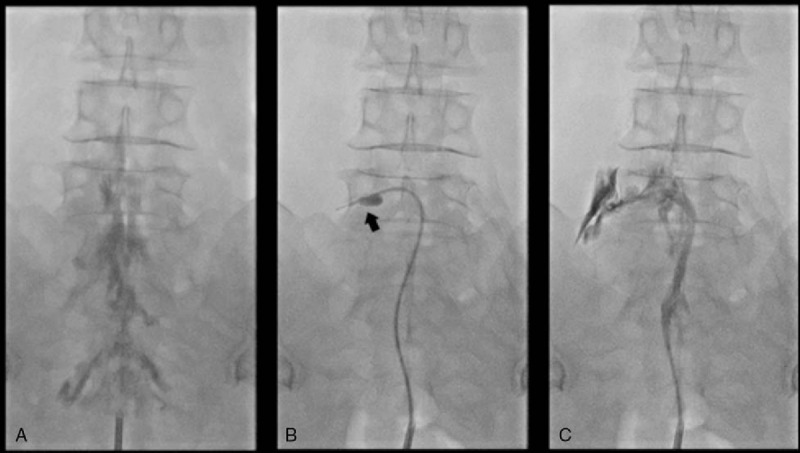
Serial fluoroscopic images of PEA combined with balloon decompression. (A) Anteroposterior view verified before the procedure showing filling defects of the contrast medium at the epidural space at both L5-S1 intervertebral foramina. (B) Fluoroscopic view showing the inflatable balloon neuroplasty catheter placed in the left L5 intervertebral foramen and the balloon filled with the contrast medium. Foraminal stenosis is visualized by the degree of balloon distortion (arrow). Decompression is performed along the intervertebral foramen by ballooning. (C) After balloon decompression and PEA along the pass from the lateral recess to the intervertebral foramen, the contrast agent spread well. PEA = percutaneous epidural adhesiolysis.
2.3. Outcome assessments and follow-up
Before the procedure was completed, all patients were taught to use an 11-point NRS (0 = no pain and 10 = worst possible pain) to assess the intensity of leg or lower back pain,[14] along with the Korean version of the Oswestry disability index (ODI) questionnaire (10 item; range, 0–100; 0 = no disability) to assess physical function.[15,16] Furthermore, the Beck depression inventory (BDI) was used to assess emotional functioning,[17] and the global perceived effect of satisfaction (GPES) according to the 7-point Likert scale was used to assess patient satisfaction and improvement.[18] The changes in analgesics were measured using the medication quantification scale III (MQS),[19] and opioid use was also checked. All data were collected at baseline, and all data, except BDI, were collected at 1, 3, and 6 months after the procedure. Follow-up data and adverse events that occurred during the treatment were individually recorded. We divided all data according to 2 patient groups to assess whether PEA with balloon decompression was effective in patients who were unresponsive to the previous PEA using a balloon-less catheter: patients with intractable LSS who did not undergo previous PEA (No-PEA) and those who were unresponsive to previous PEA that was performed with balloon-less catheters such as Racz or NaviCath (Prev-PEA).
The primary outcome was the number of successful responders to the treatment in each group and during the follow-up period. A successful response was determined on the basis of the results of some previous studies with a few modifications.[17,20,21] A successful response was defined as follows:
≥50% (or ≥4 points) reduction from the baseline leg or lower back NRS score, no increase from baseline ODI, and ≥4 points on the GPES scale or
≥30% (or ≥2 points) reduction from the baseline NRS score with any one of the following criteria—simultaneous ≥30% (or ≥10 point) reduction from baseline ODI or ≥4 points on the GPES scale.
2.4. Statistical analysis
Categorical variables are presented as absolute numbers and percentages. Continuous variables are presented as means with standard deviation (SD), 95% confidence intervals, or medians with the interquartile range. All observed data were analyzed on an intent-to-treat basis, irrespective of the losses to follow-up or withdrawal from the study. Because of the loss of data that resulted from follow-up loss, for instances of dropout, including treatment failure, a linear mixed effect model (LMEM) was used to analyze continuous variables (NRS, ODI, and GPES) at baseline and at 1, 3, and 6 months after the procedure. When the variables were compared between the 2 groups using the LMEM, the fixed effect is group and the random effect is time. For strict interpretation of the results of this study, successful follower analysis was performed with consideration of all follow-up losses as treatment failures. Changes from baseline at each time point were compared using the Mann–Whitney U test. The 2 groups were compared using Student t test and χ2 test.
To assess baseline differences between successful responders and nonresponders at 6 months after the procedure, continuous variables were compared using Student t test or Mann–Whitney U test, as appropriate. Categorical data were compared using χ2 test or Fisher's exact test to assess differences between the 2 groups, as appropriate. Analyses were performed using the SPSS Statistics version 21 (SPSS, Inc., Chicago, IL). A 2-tailed P value of <.05 was considered to be statistically significant.
3. Results
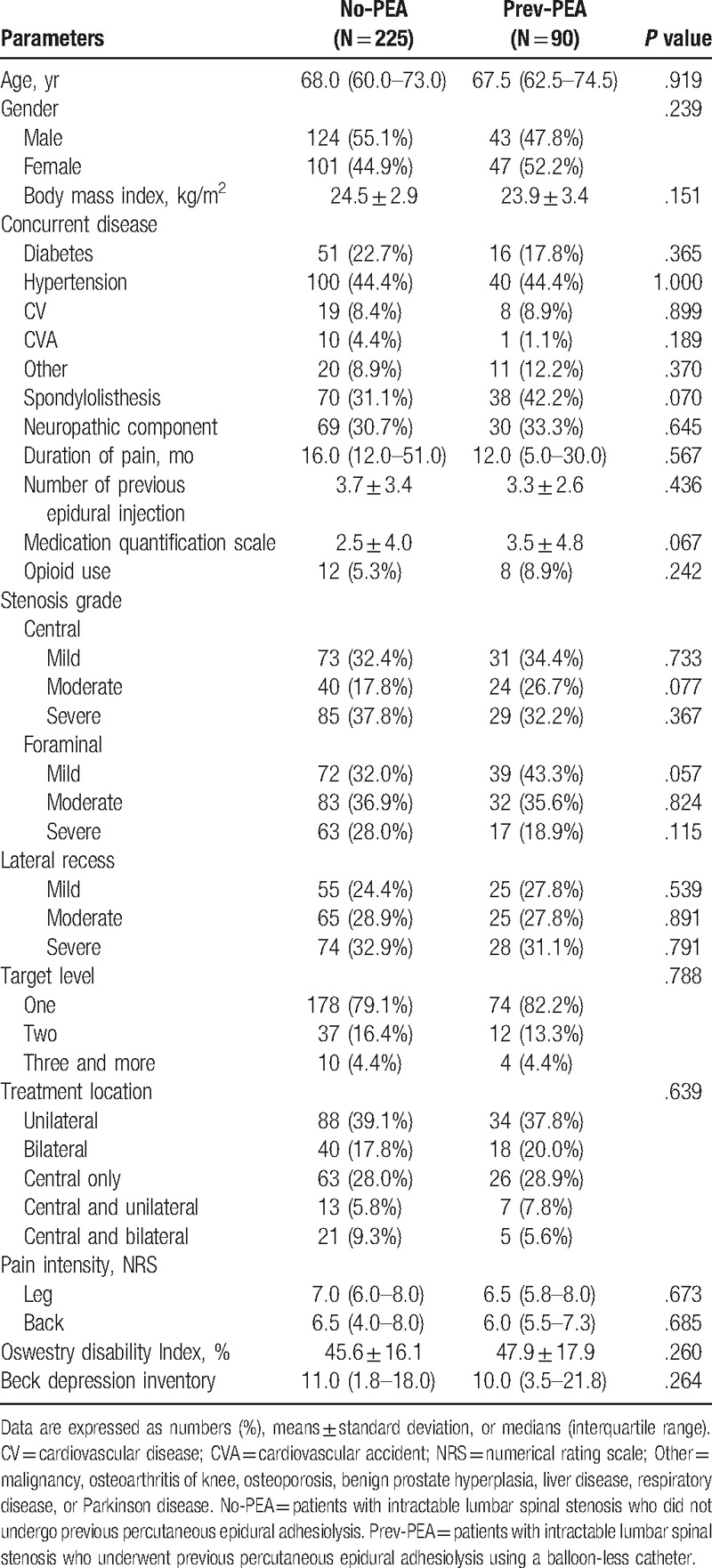
For eligibility, we screened 344 patients with LSS who were scheduled to undergo combined PEA and balloon decompression with a ZiNeu catheter between January and December 2014 at our hospital. These patients suffered from chronic lumbar radicular pain with or without lower back pain and satisfied the inclusion and exclusion criteria. Among these 344 eligible patients, 6 underwent another procedure. Moreover, 15 patients experienced complications (suspicious dura mater puncture in 12, failed ballooning because of severe adhesion in 2, and blood pressure drop in 1) during PEA and balloon decompression with a ZiNeu catheter. Baseline data for 8 patients were missing. Finally, 315 patients were included in the study (Fig. 2, Table 1). These 315 patients were divided into the No-PEA (225 patients) and Prev-PEA (90 patients) groups. The demographic characteristics and baseline data between these 2 groups were not significantly different (Table 2).
Figure 2.
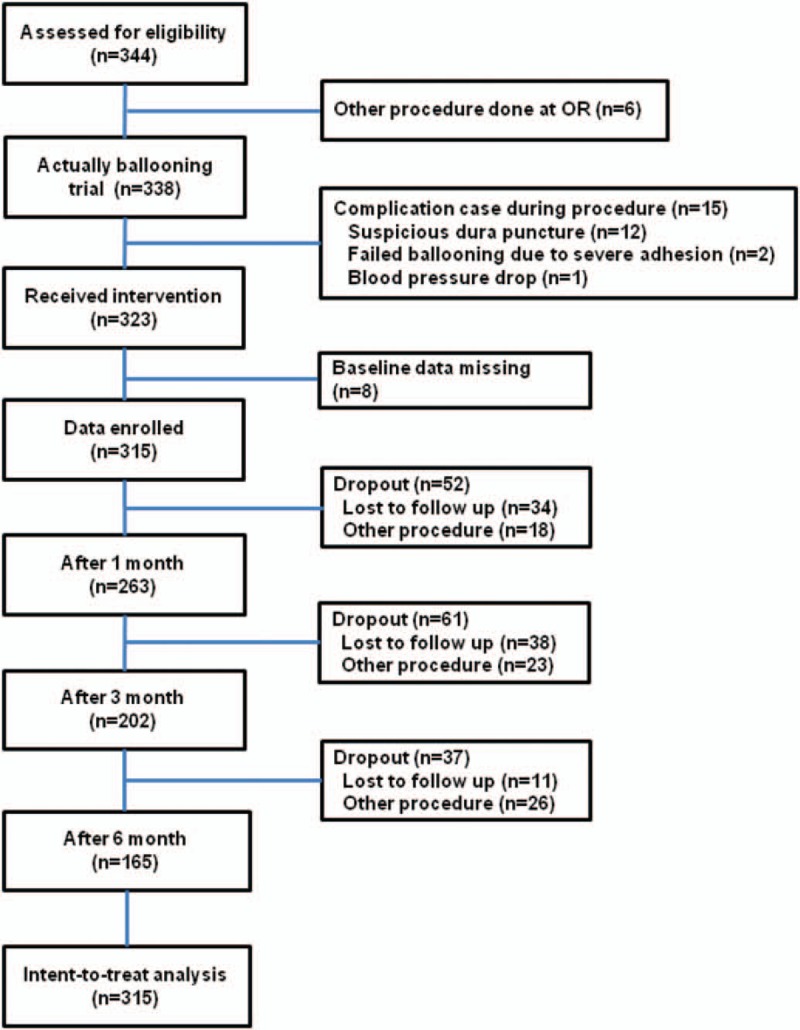
A flow diagram of the study population.
Table 1.
Baseline characteristics of the study subjects.
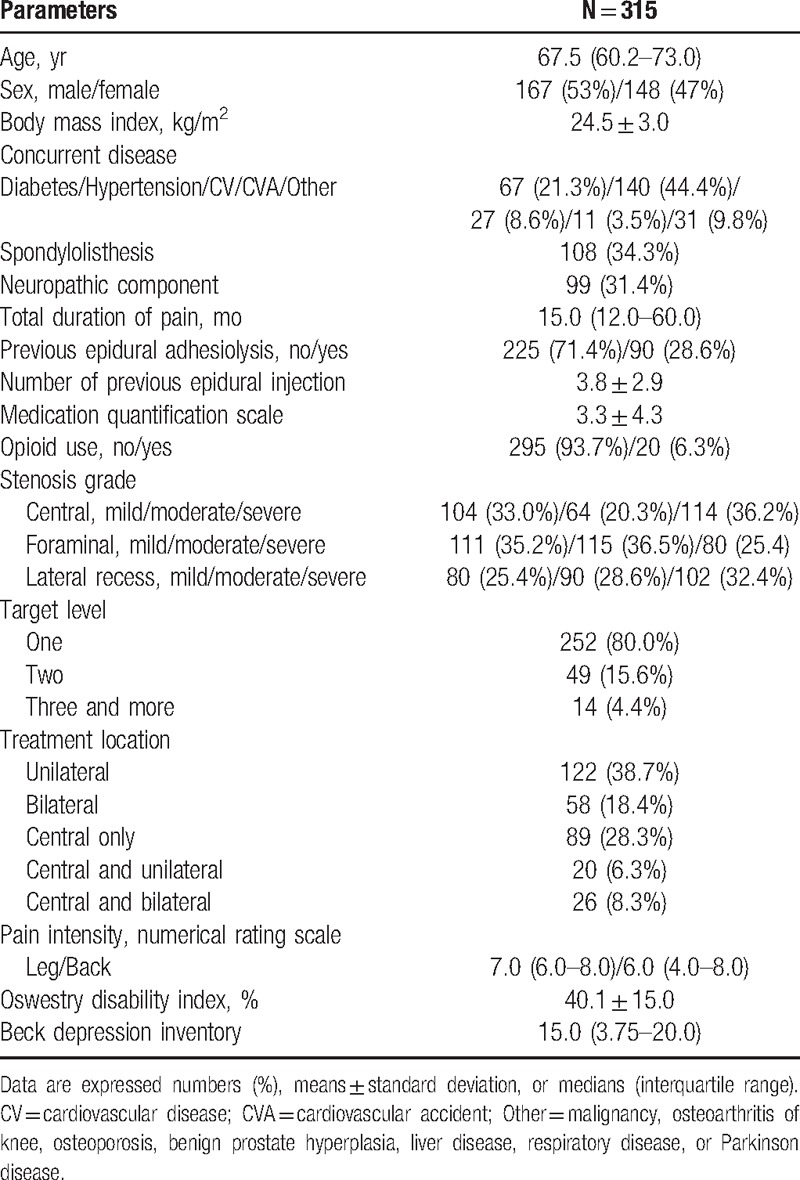
Table 2.
Characteristics of patients with intractable lumbar spinal stenosis who underwent and those who did not undergo previous percutaneous epidural adhesiolysis.
As shown in Figure 2, at 1, 3, and 6 months after the procedure, 52, 61, and 37 patients, respectively, dropped out because of loss to follow-up (34, 38, and 11, respectively) or the need for another procedure (18, 23, and 26, respectively). All of the dropout patients were considered as treatment failures.
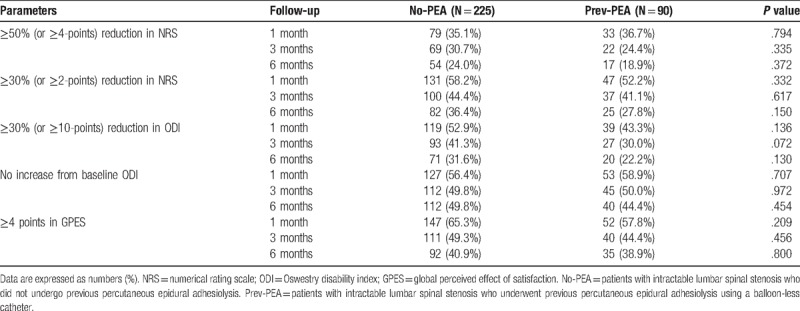
Successful treatment responses were observed at 1, 3, and 6 months in 56.4%, 42.7%, and 32.9%, respectively, of the No-PEA group and 48.9%, 37.8%, and 25.6%, respectively, of the Prev-PEA group (Table 3). The frequency of successful responders was not significantly different between the 2 groups. The observed numbers of patients in the 2 groups who satisfied the individual parameters for a successful response at each follow-up visit are listed in Table 4. No significant differences were detected between the 2 groups. MQS and opioid use were not significantly altered during the follow-up period. At 6 months, the mean MQS ± SD was 1.8 ± 3.9 in the No-PEA and 1.8 ± 3.6 in the Prev-PEA (P = .947) groups, and the numbers of patients (%) using opioid were 15 (6.7%) and 5 (5.6%), respectively (P = .804).
Table 3.
Proportions of successful responders among patients who were treated with combined decompression and adhesiolysis using an inflatable balloon catheter.
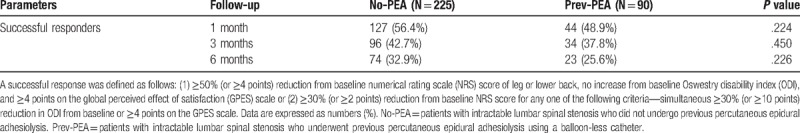
Table 4.
Observed number of patients who satisfied the individual parameters for a successful response at each follow-up visit.
The adjusted predictions of values and differences in the 2 groups from baseline for NRS of pain and ODI functional status over the 6-month follow-up period are shown in Table 5 and Figure 3. In both the groups, intent-to-treat analyses revealed a significant improvement in the mean pain score compared with that at the baseline throughout the study period (P <.001). When the back and leg pain scores were compared between the 2 groups using LMEM, no significant differences that affected the changes in NRS scores were detected between the No-PEA and Prev-PEA groups throughout the study period. P values of the interaction between the groups and time for back and leg pain were .675 and .351, respectively. The intensities of lower back and leg pain significantly improved, and this improvement was maintained following combined PEA and balloon decompression with a ZiNeu catheter during the 6-month follow-up period, irrespective of previous PEA. The functional capacity, assessed on the basis of ODI, continuously improved over the 6-month period after combined therapy in both the groups (P <.001 throughout the study period compared with that at baseline). Furthermore, the affected changes of ODI did not significantly differ between the 2 groups. P values of interaction between the groups and time for ODI were .139, except at 1 month (P = .002) after the combined procedure when the 2 groups were compared using LMEM.
Table 5.
Changes in the adjusted predictions of pain scores and physical function after combined balloon decompression and epidural adhesiolysis in patients with intractable lumbar spinal stenosis who were or were not previously treated with epidural adhesiolysis using a balloon-less catheter.
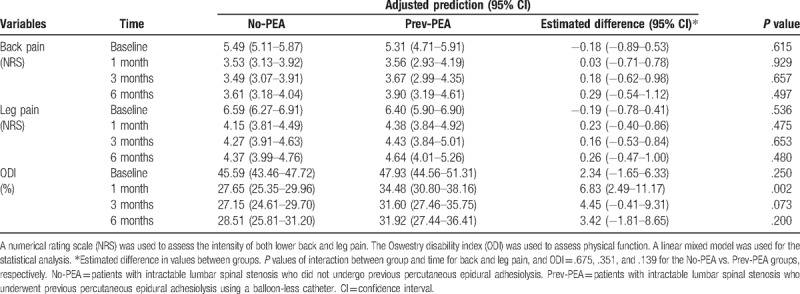
Figure 3.
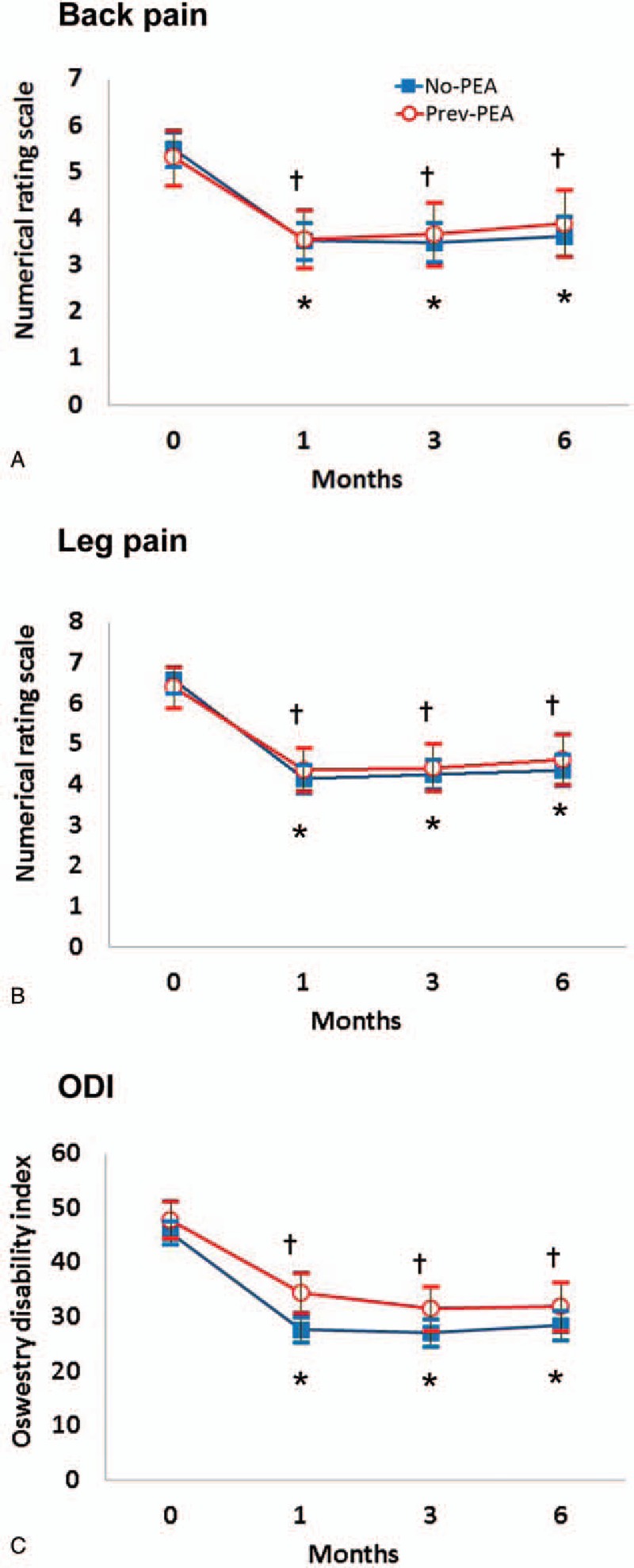
Numerical rating scale of back (A) and leg (B) pain, and the Oswestry disability index (ODI; C) at baseline (0) and 1, 3, and 6 months after combined balloon decompression and epidural adhesiolysis in the Prev-PEA and No-PEA groups. Data are presented as adjusted prediction values ± 95% confidence interval. P values of the interaction between the groups, time for back and leg pain, and ODI were .675, .351, and .139, respectively, for the No-PEA vs. Prev-PEA groups. ∗P <.001 versus baseline in the No-PEA group. †P <.001 versus baseline in the Prev-PEA group. PEA = percutaneous epidural adhesiolysis.
Although there were some complications among our study patients, such as dura mater puncture and decreases in blood pressure, no serious adverse events were noted in any case, and any adverse events that occurred throughout the study period were minor and temporary. Some patients reported temporary pain during needle insertion and paresthesia during the balloon procedure, which was tolerable and did not require additional medications or discontinuation of the procedure. The balloon procedure was discontinued in 2 patients only who reported severe pain during the procedure because of severe adhesion. Several patients complained of 2 to 3 days of residual pain during the post-procedural period, but transient pain aggravation was spontaneously relieved without any neurological sequelae. No other adverse events such as infection, intravenous injection, or persistent motor or sensory impairment were noted.
4. Discussion
This study data showed that combined PEA and balloon decompression significantly reduced back and leg pain and improved the functional capacity, assessed on the basis of ODI, in both the No-PEA and Prev-PEA groups during the 6-month follow-up period after procedure. No significant differences were detected between the 2 groups. This similarity of the results in 2 groups demonstrated that combined PEA with balloon decompression was equally effective in patients who were unresponsive to previous PEA, and those who have not previously received PEA.
PEA generally is performed with a Racz catheter or NaviCath in patients who fail to respond to conventional ESI.[7,8] However, the long-term effects of this procedure remain uncertain and controversial. PEA using a Racz-type catheter does not result in true mechanical lysis of adhesions.[22] To help accomplish this procedure, a more “steerable” catheter was developed to directly separate the adhesive region around the nerve.[23] The NaviCath showed clinical effectiveness in patients with chronic low back pain who were unresponsive to transforaminal ESI.[23,24] However, some patients with severe adhesions cannot achieve satisfactory results after PEA using the NaviCath because positioning these catheters at the target site is difficult. We evaluated whether the combined treatment with a ZiNeu catheter for PEA and decompression resulted in sufficient pain relief in patients with chronic refractory LSS and found that such pain improvement was maintained for 6 months. These patients also showed functional improvements over the 6-month period after the balloon procedure. There was an effect even in patients in whom previous PEA procedures were unsuccessful. It is notable that this procedure was effective in these difficult-to-treat patients. Therefore, we suggested that a combined treatment with a ZiNeu catheter for PEA and decompression may represent a useful alternative for overcoming the limitations of preexisting PEA procedures. In fact, balloon PEA, which incorporates balloon decompression to the currently performed PEA procedure, is a more advanced treatment than PEA utilizing a Racz catheter. For this reason, all subject to Racz catheter can be the subject of a combined balloon PEA. Because balloon PEA is more advanced than Racz-utilizing PEA, as demonstrated in the present study, balloon PEA is a potentially effective alternative in patients who did not achieve improvement with PEA using a Racz catheter.
A previous prospective observational study demonstrated that combined treatment with a balloon-inflatable ZiNeu catheter for PEA and balloon decompression resulted in sufficient pain relief and functional improvement for 12 months in patients with chronic refractory LSS.[12] A successful response was noted at 1, 3, 6, and 12 months in 72.1%, 60.7%, 57.4%, and 36.1%, respectively, of patients in the previous prospective study. Although our percentages of patients with a successful response were somewhat lower than those reported in the previous study, the trend of treatment outcomes had a similar pattern. The lower percentages of successful responders in our study may be because of the study's retrospective design. Our retrospective study had a relatively high dropout rate, with the patients being considered as treatment failures. There is a possibility that some of the patients lost to follow-up may have had a successful response.
The observed effects of the combined procedure on pain relief and functional improvement may be explained by several factors. First, combined balloon decompression and PEA using a ZiNeu catheter may increase the marginal space of the stenotic area. Some studies have suggested that circulatory disturbances, such as venous congestion, stasis, or arterial ischemia, are essential factors for radicular pain.[25,26] Combined mechanical balloon decompression and PEA of the stenotic lesion using a ZiNeu catheter can reduce circulatory disturbances. Three-dimensional reconstructed images of the epidural space have already shown that the balloon treatment increased the epidural space in the stenotic region of the intervertebral foramen.[12,13] Moreover, a previously reported randomized study showed that balloon treatment led to improvement in claudication distance, as well as physical function.[13] Second, intermittent balloon inflation/deflation exerts a major force that results in the distension of the epidural space and mechanical detachment of perineural adhesions.[13] Fibrosis and adhesions in the epidural space may develop because of inflammation around the nerve[27] and may then cause radiculopathy by interfering with the mobility of the dural sleeve of the nerve roots.[28] Nerve root mobility may have a role in the long-lasting pain relief and functional improvement. Third, because of its maneuverability and ballooning, the ZiNeu catheter can be used more effectively to deliver drugs to target regions. The ZiNeu catheter can be manipulated vertically and side-to-side. This maneuverability allows physicians to place the catheter more easily at target lesions, such as a lateral recess, intervertebral foramen, or extraforaminal space. Moreover, the thin epidural catheter can be maintained at the target site to enable epidural drug injections for several days. Therefore, this combined procedure may relieve severe adhesions more effectively and enable localized drug delivery to target lesion sites.[4,11] Recently, it has also been reported that factors associated with the outcome of the balloon treatment in patients with intractable lumbar spinal stenosis. Diabetes and co-existing lower back pain may be poor prognostic factors of the combined balloon decompression and PEA.[12] In addition, it was shown that lumbar foraminal stenosis mainly caused by degenerative disc herniation was an independent factor associated with successful responses of the balloon treatment.[29,30] Taken together with the present findings, suggested indications of the combined balloon decompression and PEA are followings:
-
1)
chronic refractory lumbar radicular leg pain with neurogenic claudication;
-
2)
failure or short duration of effects to other interventional procedures including PEA without balloon-less catheter;
-
3)
lumbar spinal stenosis caused primarily by degenerative disc herniation;
-
4)
perineural adhesion by chronic degenerative disc.
This study had several limitations. First, our combined intervention was a complex treatment that comprised several components such as administering various drugs, mechanical adhesiolysis, flushing with saline, and ballooning. Although we aimed to exclude other factors that could affect our results, we could not rule out the possibility that other components of our complex treatment provided the essential therapeutic effect. Second, the definition of a successful response can be variable. We established the definition of a successful response based on several previous reports and recommendations,[17,20,21] although it remains imperfect. We carefully selected the response criteria to reflect treatment success as either substantial or clinically meaningful pain relief (NRS) combined with patient-reported outcomes, which included ODI and GPES.[14,16] The definition of a successful response must include multiple factors such as pain relief, functional and emotional factors, previous procedure history and medications, because the perception of pain is influenced by various factors. In our study, we could not include all the factors associated with a successful response. If the definition was changed, we may have obtained different results. Third, our study had a retrospective design, and many dropout cases were considered as treatment failures. Approximately half of the study patients had dropped out by the 6-month follow-up visit. The patients who underwent another procedure during the study period were also considered as treatment failures; however, they may not have actually been treatment failures. Therefore, randomized controlled trials to assess the effects of this treatment modality are warranted, with careful and proper selection criteria being applied. In fact, in a recent randomized controlled trial with combined PEA and balloon decompression, the percentage of successful responders at 6 months was 58.3%.[31] Finally, this retrospective study had no control group. Combined balloon decompression and PEA using a ZiNeu catheter were performed in all patients in our study. However, a randomized prospective trial with an active control group is currently ongoing at our center to assess the effects of this treatment.
In conclusion, combined treatment with balloon decompression and epidural adhesiolysis using a ZiNeu catheter can lead to significant pain relief and functional improvement for at least 6 months. The present procedure may be effective in patients with both chronic radicular pain and neurogenic claudication intractable to conventional interventional treatment. This approach is also feasible for patients with intractable LSS who did not undergo previous PEA and may also benefit patients in whom previous conventional PEA using a Racz catheter or NaviCath failed. Therefore, this treatment modality can be a useful alternative to overcome the limitations of preexisting epidural adhesiolysis procedures.
Acknowledgments
The authors acknowledge the contribution to recruitment and data collection of Pain Clinic members of Asan Medical Center. The authors would like to thank Enago (http://www.enago.co.kr) for the English language review.
Author contributions
Conceptualization: Seong-Sik Cho, Seong-Soo Choi.
Data curation: Myong-Hwan Karm, Syn-Hae Yoon, Dong-Kyun Seo, Sookyung Lee.
Formal analysis: Myong-Hwan Karm, Seong-Sik Cho, Seong-Soo Choi.
Supervision: Seong-Sik Cho, Seong-Soo Choi.
Writing – Original Draft: Myong-Hwan Karm.
Writing – Review & Editing: Yongsoo Lee, Seong-Sik Cho, Seong-Soo Choi.
Seong-Soo Choi orcid: 0000-0002-2333-0235.
Footnotes
Abbreviations: BDI = Beck depression inventory, ESI = epidural steroid injection, GPES = global perceived effect of satisfaction, LMEM = linear mixed effect model, LSS = lumbar spinal stenosis, MQS = medication quantification scale III, MRI = magnetic resonance imaging, NRS = numerical rating scale, ODI = Oswestry disability index, PEA = percutaneous epidural adhesiolysis, SD = standard deviation.
S-So.C and S-Si.C contributed equally to this work.
This work was presented in part as M-HK's MS thesis at the University of Ulsan College of Medicine (2015).
The authors have no conflicts of interest to disclose.
References
- [1].Benoist M. The natural history of lumbar degenerative spinal stenosis. Joint Bone Spine 2002;69:450–7. [DOI] [PubMed] [Google Scholar]
- [2].Atlas SJ, Keller RB, Wu YA, et al. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the maine lumbar spine study. Spine 1976;30:936–43. [DOI] [PubMed] [Google Scholar]
- [3].Manchikanti L, Abdi S, Atluri S, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician 2013;16suppl 2:S49–283. [PubMed] [Google Scholar]
- [4].Lee F, Jamison DE, Hurley RW, et al. Epidural lysis of adhesions. Korean J Pain 2014;27:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Helm S, Racz GB, Gerdesmeyer L, et al. Percutaneous and endoscopic adhesiolysis in managing low back and lower extremity pain: a systematic review and meta-analysis. Pain Physician 2016;19:E245–282. [PubMed] [Google Scholar]
- [6].Jamison DE, Hsu E, Cohen SP. Epidural adhesiolysis: an evidence-based review. J Neurosurg Sci 2014;58:65–76. [PubMed] [Google Scholar]
- [7].Lee JH, Lee SH. Clinical effectiveness of percutaneous adhesiolysis versus transforaminal epidural steroid injection in patients with postlumbar surgery syndrome. Reg Anesth Pain Med 2014;39:214–8. [DOI] [PubMed] [Google Scholar]
- [8].Manchikanti L, Bakhit CE. Percutaneous lysis of epidural adhesions. Pain Physician 2000;3:46–64. [PubMed] [Google Scholar]
- [9].Gerdesmeyer L, Wagenpfeil S, Birkenmaier C, et al. Percutaneous epidural lysis of adhesions in chronic lumbar radicular pain: a randomized, double-blind, placebo-controlled trial. Pain Physician 2013;16:185–96. [PubMed] [Google Scholar]
- [10].Bosscher HA, Heavner JE, Grozdanov P, et al. The peridural membrane of the human spine is well innervated. Anat Rec 2016;299:484–91. [DOI] [PubMed] [Google Scholar]
- [11].Choi SS, Joo EY, Hwang BS, et al. A novel balloon-inflatable catheter for percutaneous epidural adhesiolysis and decompression. Korean J Pain 2014;27:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Choi SS, Lee JH, Kim D, et al. Effectiveness and factors associated with epidural decompression and adhesiolysis using a balloon-inflatable catheter in chronic lumbar spinal stenosis: 1-year follow-up. Pain Med 2016;17:476–87. [DOI] [PubMed] [Google Scholar]
- [13].Kim SH, Choi WJ, Suh JH, et al. Effects of transforaminal balloon treatment in patients with lumbar foraminal stenosis: a randomized, controlled, double-blind trial. Pain Physician 2013;16:213–24. [PubMed] [Google Scholar]
- [14].Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- [15].Kim DY, Lee SH, Lee HY, et al. Validation of the Korean version of the oswestry disability index. Spine 2005;30:E123–127. [DOI] [PubMed] [Google Scholar]
- [16].Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine 2008;33:90–4. [DOI] [PubMed] [Google Scholar]
- [17].Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- [18].Van Zundert J, Patijn J, Kessels A, et al. Pulsed radiofrequency adjacent to the cervical dorsal root ganglion in chronic cervical radicular pain: a double blind sham controlled randomized clinical trial. Pain 2007;127:173–82. [DOI] [PubMed] [Google Scholar]
- [19].Gallizzi M, Gagnon C, Harden RN, et al. Medication Quantification Scale Version III: internal validation of detriment weights using a chronic pain population. Pain Pract 2008;8:1–4. [DOI] [PubMed] [Google Scholar]
- [20].Geurts JW, van Wijk RM, Wynne HJ, et al. Radiofrequency lesioning of dorsal root ganglia for chronic lumbosacral radicular pain: a randomised, double-blind, controlled trial. Lancet 2003;361:21–6. [DOI] [PubMed] [Google Scholar]
- [21].Koh W, Choi SS, Karm MH, et al. Treatment of chronic lumbosacral radicular pain using adjuvant pulsed radiofrequency: a randomized controlled study. Pain Med 2015;16:432–41. [DOI] [PubMed] [Google Scholar]
- [22].Birkenmaier C, Baumert S, Schroeder C, et al. A biomechanical evaluation of the epidural neurolysis procedure. Pain Physician 2012;15:E89–97. [PubMed] [Google Scholar]
- [23].Lee JH, Lee SH. Clinical effectiveness of percutaneous adhesiolysis using Navicath for the management of chronic pain due to lumbosacral disc herniation. Pain Physician 2012;15:213–21. [PubMed] [Google Scholar]
- [24].Lee JH, Lee SH. Clinical effectiveness of percutaneous adhesiolysis and predictive factors of treatment efficacy in patients with lumbosacral spinal stenosis. Pain Med 2013;14:1497–504. [DOI] [PubMed] [Google Scholar]
- [25].Berthelot JM, Le Goff B, Maugars Y. The role for radicular veins in nerve root pain is underestimated: limitations of imaging studies. Joint Bone Spine 2011;78:115–7. [DOI] [PubMed] [Google Scholar]
- [26].Kobayashi S, Takeno K, Miyazaki T, et al. Effects of arterial ischemia and venous congestion on the lumbar nerve root in dogs. J Orthop Res 2008;26:1533–40. [DOI] [PubMed] [Google Scholar]
- [27].Rydevik B, Brown MD, Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine 1984;9:7–15. [DOI] [PubMed] [Google Scholar]
- [28].Merrild U, Sogaard I. Sciatica caused by perifibrosis of the sciatic nerve. J Bone Joint Surg Br 1986;68:706. [DOI] [PubMed] [Google Scholar]
- [29].Kim D, Cho SS, Moon YJ, et al. Factors associated with successful responses to transforaminal balloon adhesiolysis for chronic lumbar foraminal stenosis: a retrospective study. Pain Physician 2017;20:E841–848. [PubMed] [Google Scholar]
- [30].Seo DK, Lee S, Lee G, et al. Retrodiscal epidural balloon adhesiolysis through kambin's triangle in chronic lumbar spinal stenosis: a retrospective analysis and technical considerations. Medicine (Baltimore) 2018;97:e12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Karm MH, Choi SS, Kim DH, et al. Percutaneous epidural adhesiolysis using inflatable balloon catheter and balloon-less catheter in central lumbar spinal stenosis with neurogenic claudication: a randomized controlled trial. Pain Physicain 2018;21:593–606. [PubMed] [Google Scholar]


