Abstract
The neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) are not fully evaluated for the diagnosis of musculoskeletal tumors, especially spine tumors. The objective of our study was to assess the feasibility of NLR and PLR as indicators for pretreatment diagnosis of spine tumors.
Patients who underwent surgical treatment in our hospital for spine tumors were retrospectively analyzed. Blood test results (neutrophil, lymphocyte, and platelet counts) and final pathological results from surgery or biopsy specimen were collected. Spine tumors were divided into 4 groups. Diagnostic values of NLR and PLR were analyzed using the area under the receiver operating characteristic (ROC) curve (AUC).
There were 503 patients included. The average age of all patients was 46.3 years. Age, NLR, and PLR were significantly different between benign and malignant tumors groups (P < .05), and ROC analysis showed that the AUC was 0.704 and 0.637 for NLR and PLR. Age, location, NLR, and PLR were significantly different between primary and nonprimary tumor groups (P < .05), and ROC analysis showed that the AUC was 0.713 and 0.647 for NLR and PLR. Age, location, NLR, and PLR were significantly different between primary benign and primary malignant tumor groups (P < .05), and ROC analysis showed that the AUC was 0.624 and 0.577 for NLR and PLR.
Pretreatment NLR and PLR had clinical significance in the identification and pretreatment diagnosis of spine tumors. Additionally, NLR and PLR were significantly different between benign and malignant tumors, primary and nonprimary tumors, and primary benign and primary malignant tumors.
Keywords: diagnosis, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, spine tumor
1. Introduction
Spine tumors, including primary and metastatic tumors, are relatively rare among spine disorders; however, they could lead to the destruction of bone, resulting in fractures, pain, and neurological deficits.[1–8] Pretreatment diagnosis of spine tumors is important because the choice of treatment depends largely on the characteristics of the tumor.[1,3,9,10] The comprehensive application of diagnostic techniques, including x-ray, computed tomography (CT), magnetic resonance imaging, and bone scintigraphy, have made it easier to detect spine tumors, although they are inadequate for determining the sources of tumors. Positron emission tomography-CT and percutaneous biopsy may be more helpful; however, they are expensive and invasive.[11–13]
It is increasingly evident that tumor-infiltrating inflammatory cells, mediators, and cytokines play important roles in tumor growth, invasion, and metastasis.[14] Accordingly, levels of serum white blood cells, neutrophils, lymphocytes, and platelets, as well as the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR), have been used as prognostic markers for many cancers.[15–20] In addition, several studies have shown a significant association between higher NLR or PLR and poor survival.[21–23]
Nevertheless, only a few studies have investigated the prognostic value of NLR and PLR for musculoskeletal tumors.[24,25] Additionally, the significance of inflammatory markers for the diagnosis of musculoskeletal tumors, especially spine tumors, has not been evaluated. The objective of our study was to assess the feasibility of the inflammatory markers, NLR and PLR, to function as indicators for pre-treatment diagnosis of spine tumors.
2. Materials and methods
The hypothesis we hold is that pretreatment NLR and PLR had clinical significance in the identification and pre-treatment diagnosis of spine tumors. This study was approved by our hospital's ethics committee and conducted according to the principles of the Declaration of Helsinki.
Inclusion criteria were as follows:
-
(1)
all spine tumors were pathologically confirmed with a specific type,
-
(2)
blood samples were obtained before operation and/or treatment,
-
(3)
patients did not receive any treatment before the blood tests, and
-
(4)
the osseous structures and/or neurostructures of the spine were affected by the tumors.
Exclusion criteria were as follows:
-
(1)
patients with a fever above 38°C at admission suggesting a possible infection,
-
(2)
patients diagnosed with spinal tuberculosis and nonspecific inflammation according to the final pathological reports,
-
(3)
patients under 6 years old with unstable blood test results,[26]
-
(4)
patients with an exact history of autoimmune and/or hematological diseases,
-
(5)
patients with ambiguous pathological reports, and
-
(6)
patients with intramedullary and/or subdural tumors.
As our hospital is one of only a few medical centers in our country to have a surgical unit specializing in the spine tumors, 544 patients who underwent surgical treatment in our hospital for spine tumors between 2012 and 2016 were retrospectively analyzed. Based on the inclusion and exclusion criteria, 503 patients were finally enrolled.
Patients routinely had peripheral blood tests when visiting the hospital. Also, CT-guided biopsy was recommended for patients with difficult-to-diagnose spinal-occupying tumors. The following data were collected: age; sex; tumor location; blood test results, which included neutrophil, lymphocyte, and platelet counts; and the final pathological results from the surgery or biopsy specimen. For tumors with multiple-level involvements and/or tumors that were located in the junctional levels of the spine (eg, C7-T1 and T12-L1), the location was determined according to the major part (volume and center) of the tumor. The NLR was defined as neutrophil count divided by lymphocyte count, and the PLR as platelet count divided by lymphocyte count.
For analysis, all spine tumors were divided into 4 groups based on the World Health Organization classification of tumors[27]: primary spine benign tumors, primary spine malignant tumors, spine metastasis tumors, and spine hematological lesions.
2.1. Statistical analyses
SPSS version 20.0 (IBM Inc, Chicago, IL) was used to analyze collected data. The Kolmogorov–Smirnov test was applied to identify whether the data were normally distributed. For measurable variables, the mean value and standard deviation were presented if the data were normally distributed or the median and range values were presented. Student t test was used to compare differences in normally distributed data, and the Mann–Whitney U test was used for non-normally distributed data. For immeasurable variables, we used percentages to present the data and the chi-square test or Fisher exact test to compare the differences. The diagnostic values of NLR and PLR were analyzed by the receiver operating characteristic curve (ROC) and the area under the ROC curve (AUC). Finally, the Youden index was used to determine the cut-off value. The statistical significance level was set at P < .05.
3. Results
3.1. General profiles
A total of 503 patients were included in this study, including 288 men and 215 women (male-to-female ratio, 1.34:1). The average age of all the patients was 46.3 years (range, 6–84 years). Tumors were located in the cervical, thoracic, lumbar, and sacrum spine in 224 (44.5%), 177 (35.2%), 80 (15.9%), and 22 (4.4%) cases, respectively.
3.2. Characteristics of the different tumor groups
Based on the final pathological reports, we divided the patients into 4 groups.
3.2.1. Primary spine benign tumor group
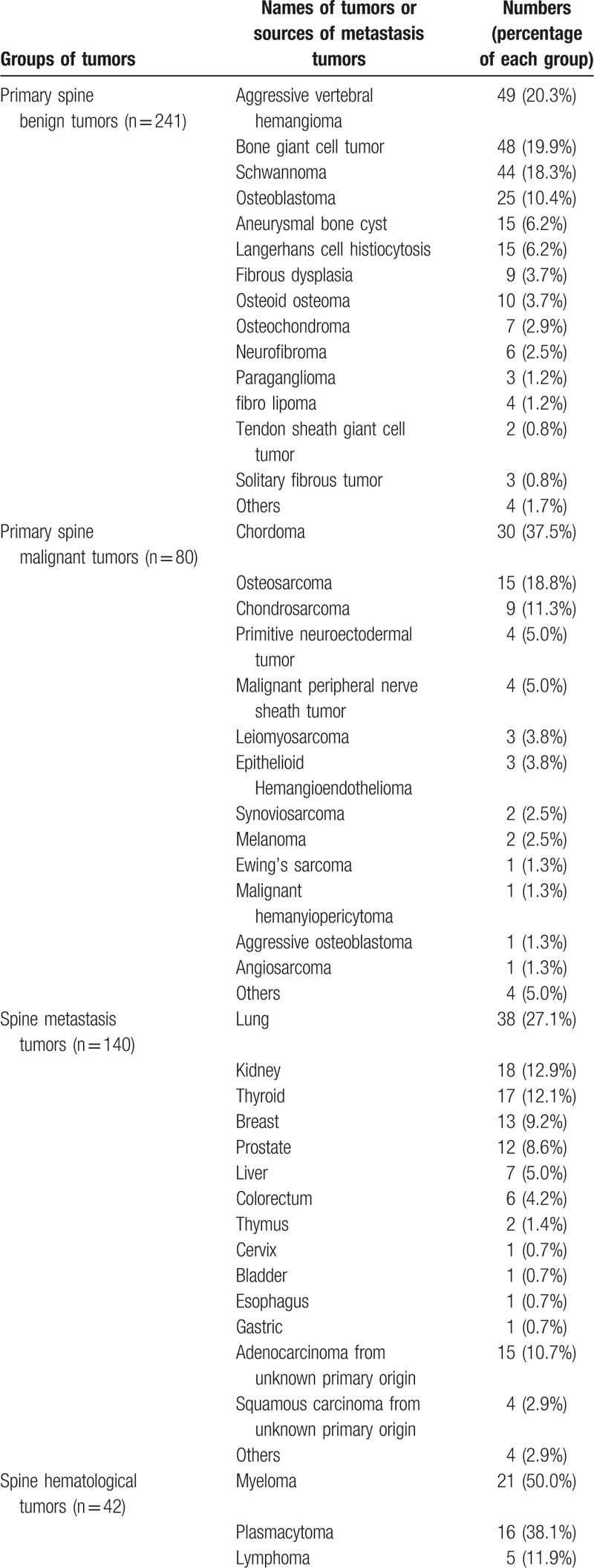
Two hundred forty-one patients, including 130 men and 111 women (male-to-female ratio, 1.17:1), were included in the primary spine benign tumor group. The average age was 36.2 years (range, 6–76 years). Primary benign tumors were located in the cervical, thoracic, lumbar, and sacrum spine in 109 (45.2%), 84 (34.9%), 38 (15.8%), and 10 (4.1%) cases, respectively. Seventy-four patients (30.7%) had multiple-level involvements. The most common types of primary spine benign tumors were aggressive vertebral hemangioma (20.3%), bone giant cell tumor (19.9%), schwannoma (18.3%), osteoblastoma (10.4%), and aneurysmal bone cyst (6.2%).
3.2.2. Primary spine malignant tumor group
There were 80 patients in the primary spine malignant tumor group, including 50 men and 30 women (male-to-female ratio, 1.67:1). The average age was 46.8 years (range, 6–76 years). Tumors were located in the cervical, thoracic, lumbar, and sacrum spine in 41 (51.3%), 19 (23.8%), 10 (12.5%), and 10 (12.5%) cases, respectively. Thirty-three patients (41.3%) had multiple-level involvements. Chordoma (37.5%), osteosarcoma (18.8%), and chondrosarcoma (11.3%) were the most common types of spine malignant tumors.
3.2.3. Spine metastasis tumor group
There were 140 patients in the spine metastasis tumor group, including 86 men and 54 women (male-to-female ratio, 1.59:1). The average age was 60.5 years (range, 21–84 years). Tumors were located in the cervical, thoracic, lumbar, and sacrum spine in 57 (40.7%), 57 (40.7%), 24 (17.1%), and 2 (1.4%) cases, respectively. Fifty-one patients (36.4%) had multiple-level involvements. Of all the metastatic spinal tumors, the lung (27.1%), kidney (12.9%), thyroid (12.1%), breast (9.2%), and prostate (8.6%) were the most common sources of spinal metastatic tumors. Additionally, some patients had tumors of unknown primary origins (adenocarcinoma and squamous carcinoma in 10.7% and 2.9% of the patients, respectively).
3.2.4. Spine hematological tumor group
There were 42 patients in the spine hematological tumor group, including 22 men and 20 women (male-to-female ratio, 1.1:1). The average age was 55.9 years (range, 16–76). Tumors were located in the cervical, thoracic, lumbar, and sacrum spine in 17 (40.5%), 17 (40.5%), 8 (19.0%), and 0 (0%) cases. Twelve patients (28.6%) had multiple-level involvements.
The types of all of the tumors and the sources of metastasis tumors are listed in Table 1.
Table 1.
The summary of 503 spine tumors.
3.3. Comparison between groups and the significance of NLR and PLR
To evaluate the significance of NLR and PLR, we made several intergroup comparisons.
3.4. Benign versus malignant tumors
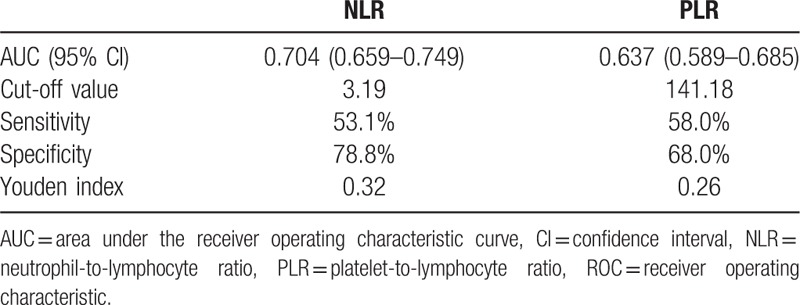
Benign tumors were defined as primary spine benign tumors. Malignant tumors included primary spine malignant tumors, spine metastasis tumors, and spine hematological tumors. The detailed comparison is presented in Table 2. Age, NLR, and PLR were significantly different between the 2 groups (P < .001). The ROC analysis (Fig. 1 and Table 3) showed that the AUC of NLR was 0.704 (95% confidence interval [CI]: 0.659–0.749) and the AUC of PLR was 0.637 (95% CI: 0.589–0.685). The best cut-off values for NLR and PLR by Youden index were 3.19 and 141.18, respectively.
Table 2.
Comparations between spine benign and malignant tumor groups.
Figure 1.
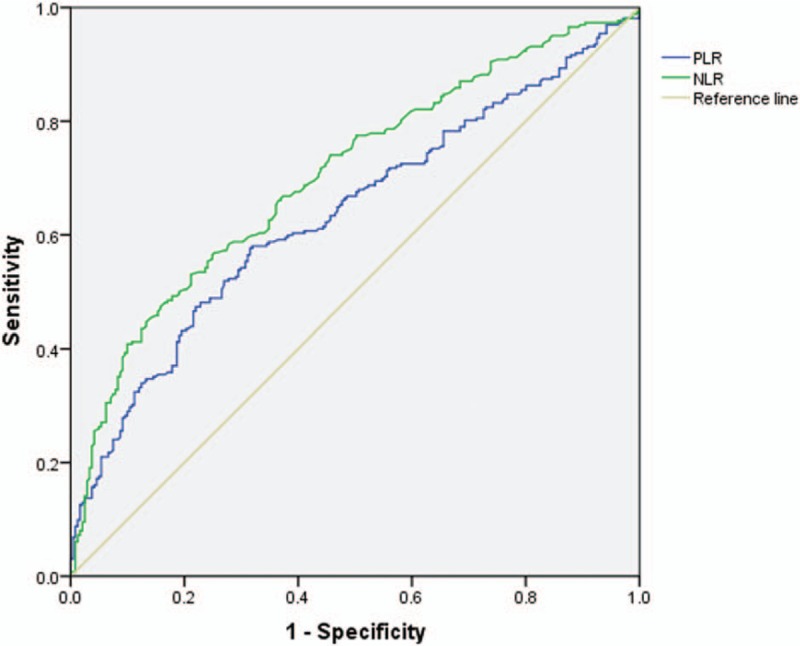
The ROC analysis of NLR and PLR between benign and malignant spine groups. The AUC for NLR was 0.704, for PLR was 0.637. AUC = area under the receiver operating characteristic curve, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio, ROC = receiver operating characteristic.
Table 3.
The ROC analysis of NLR and PLR between spine benign and malignant tumor groups.
3.5. Primary versus nonprimary tumors
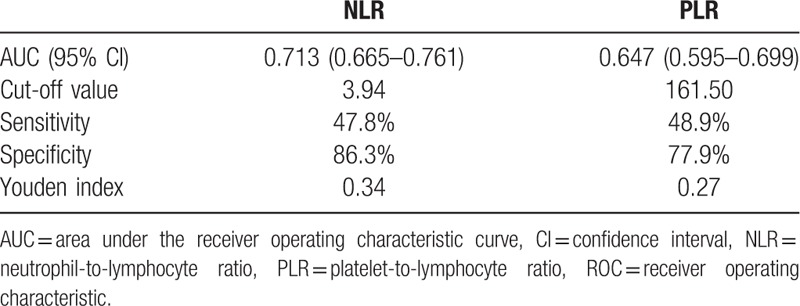
Primary tumors were defined as primary spine benign tumors and primary malignant tumors. Nonprimary tumors included spine metastasis tumors and spine hematological tumors. The detailed comparison is presented in Table 4. Age, location, NLR, and PLR were significantly different between the 2 groups (P < .05). The ROC analysis (Fig. 2 and Table 5) showed that the AUC of NLR was 0.713 (95% CI: 0.665–0.761) and the AUC of PLR was 0.647 (95% CI: 0.595–0.699). The best cut-off values for NLR and PLR by Youden index were 3.94 and 161.50, respectively.
Table 4.
Comparations between primary and non-primary spine tumor groups.
Figure 2.
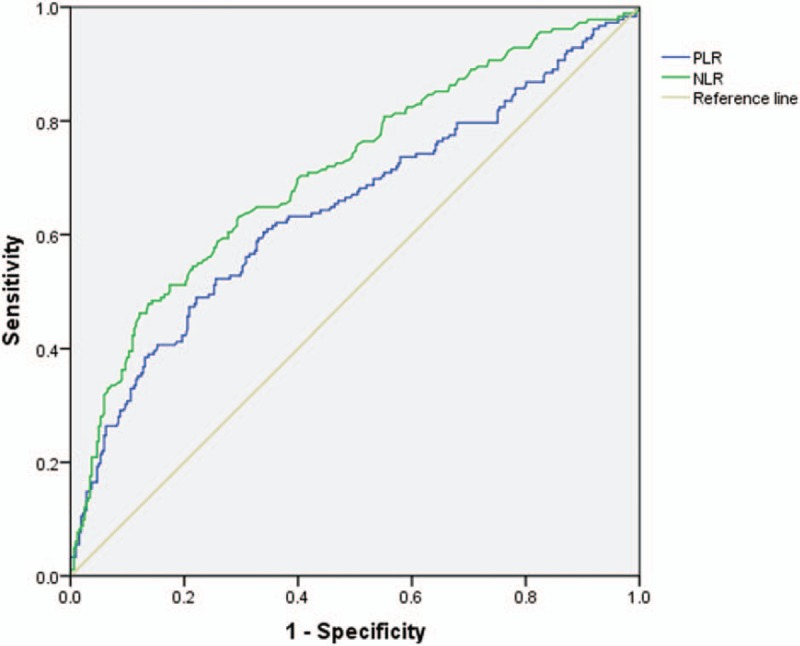
The ROC analysis of NLR and PLR between primary and nonprimary spine tumor groups. The AUC for NLR was 0.713, for PLR was 0.647. AUC = area under the receiver operating characteristic curve, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio, ROC = receiver operating characteristic.
Table 5.
The ROC analysis of NLR and PLR between primary and nonprimary spine tumor groups.
3.6. Primary benign versus primary malignant tumors
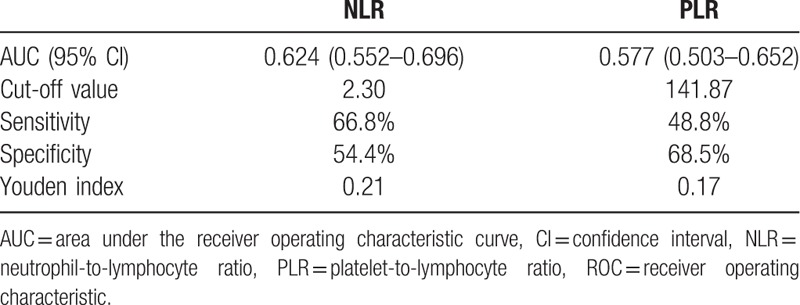
Finally, we compared primary benign and primary malignant tumors. The detailed comparison is presented in Table 6. Age, location, NLR, and PLR were significantly different between the 2 groups (P < .05). The ROC analysis (Fig. 3 and Table 7) showed that the AUC of NLR was 0.624 (95% CI: 0.552–0.696) and the AUC of PLR was 0.577 (95% CI: 0.503–0.652). The best cut-off values for NLR and PLR by Youden index were 2.30 and 141.87, respectively.
Table 6.
Comparations between primary benign malignant spine tumor groups.
Figure 3.
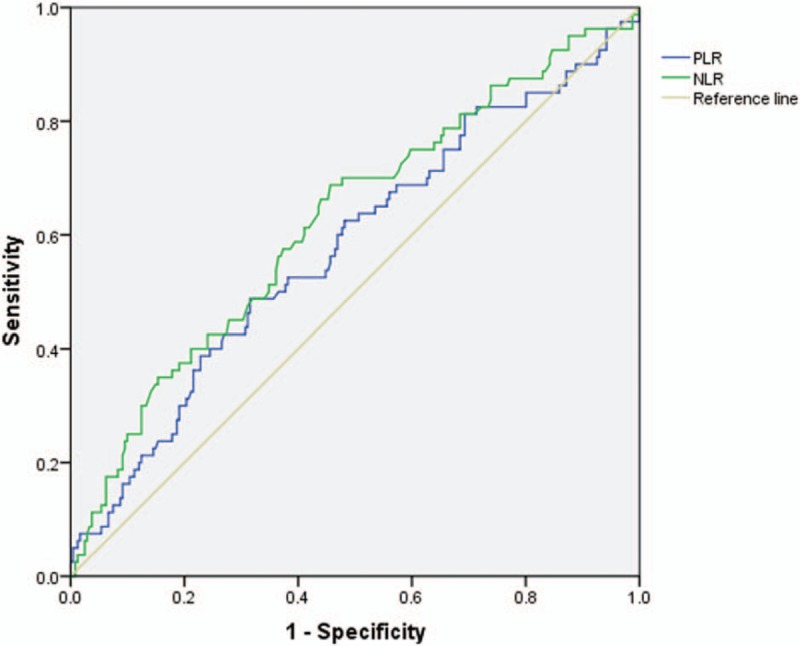
The ROC analysis of NLR and PLR between primary benign and malignant spine tumor groups. The AUC for NLR was 0.624, for PLR was 0.577. AUC = area under the receiver operating characteristic curve, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio, ROC = receiver operating characteristic.
Table 7.
The ROC analysis of NLR and PLR between primary benign and malignant spine tumor groups.
4. Discussion
Spine tumors are rare clinical conditions that can have catastrophic consequences when causing neurological deficits. Most published studies of spine tumors are small cases series. In our study, we reviewed a relatively large number of patients (n = 503) with different types of spine tumors. Additionally, we explored the significance of pretreatment NLR and PLR in distinguishing different types of spine tumors. To the best of our knowledge, this has not been reported in the literature.
Inflammation plays an important role in tumor growth, invasion, angiogenesis, and metastasis[28] and is recognized as a characteristic feature of tumor development and progression.[14] Cancer-related inflammation not only refers to the local immune reaction at the site of the tumor but also systemic inflammation,[29] which includes circulating cytokines, small inflammatory proteins, circulating immune cells, and acute-phase proteins. There is evidence that the systemic inflammatory response is associated with tumor progression through genetic mutations, epigenetic modifications, tumor metastasis, and tumor cell proliferation.[30] Neutrophils play a role in tumor angiogenesis through the production of proangiogenic factors, which contribute to the adhesion and seeding of distant sites.[31] In addition, neutrophilia can restrain the immune system by inhibiting the cytolytic effects of immune cells.[32] Platelets, which can be recruited by tumor cells, can protect tumor cells from the immune reaction and facilitate their dissemination.[33] In contrast, lymphocytes, as the basic component of the adaptive and innate immune system, are essential in providing antitumor immunity. Specifically, CD4+ and CD8+ T cells recognize tumor antigens and have been proven to induce tumor cell apoptosis.[34,35] In light of this, an increased NLR, as a result of increased neutrophil counts or decreased lymphocyte counts, could reflect tumor-related systemic inflammation, similar to PLR.
Since it has been recognized that there are complex interactions between tumors and inflammatory responses,[28,36] research is increasingly focusing on the use of inflammation biomarkers to predict the behavior of tumors. Numerous clinical studies have explored the prognostic value of inflammation biomarkers such as NLR and PLR in the treatment results and prognosis of cancers, including lung cancer, hepatocellular carcinoma, colorectal cancer, breast cancer, and prostate cancer. Moreover, higher NLR and PLR are associated with poorer prognosis.[16–20,22] However, studies on the prognostic value of inflammation biomarkers for musculoskeletal tumors are inadequate. Xia et al reported the value of NLR and PLR in the prognosis of osteosarcoma; their results showed that NLR and PLR could reflect the clinical prognosis such as advanced stage and metastasis, and NLR was more predictive than PLR.[25] Li et al reported a higher NLR was related to recurrence and decreased survival in sarcomas.[37] Li et al reported NLR and PLR were independent factors for the survival of patients with spinal giant cell tumors after gross total resection.[24] In our study of 503 spine tumor patients, we showed that preoperative NLR and PLR are valuable in distinguishing benign and malignant spine tumors, primary and nonprimary spine tumors, and primary benign and primary malignant spine tumors. NLR was superior to PLR in terms of AUC. Based on a review of the literature, this is the first report on the usefulness of pretreatment inflammation biomarkers for prediagnosis of spine tumors.
However, obvious limitations regarding the application of NLR and PLR in clinical practice remain. First, blood test results are unstable and may be affected by a number of factors, including infection, medicine treatment history, age, and sex. Second, although we found statistically significant differences between the tumor groups regarding NLR and PLR, the ROC analyses were not ideal, and the AUCs were not as high as expected. Using the Youden index, the sensitivities and specificities of the cut-off values were not ideal either, which hampers the clinical applications of our results. For an accurate diagnosis of spine tumors, using NLR or PLR alone is not sufficient; thus, we recommend an integrated and combined approach using a variety of methods. In our clinical practice, we routinely perform CT-guided biopsy for difficult-to-diagnose spine tumors; the accuracy rates of CT-guide biopsies are as high as 90% based on our clinical experience and published studies.[11,13,38,39] Although the use of inflammation biomarkers such as NLR and PLR for the diagnosis of spine tumors is not currently feasible, our study provided evidence that will be useful for future research on its clinical applications and underlying biological mechanisms. Further research on NLR and PLR and its interactions with spine tumors are expected.
5. Conclusion
This study reviewed the pathological types and basic information of 503 spine tumors. Pretreatment NLR and PLR had clinical significance in the identification and pretreatment diagnosis of spine tumors. Furthermore, NLR and PLR were found to be valuable in distinguishing between benign and malignant tumors, primary and nonprimary tumors, and primary benign and primary malignant tumors.
Author contributions
Data curation: Ben Wang, Yiyuan Wang.
Formal analysis: Yan Li, Ben Wang, Siyu Zhou, Hua Zhang.
Funding acquisition: Liang Jiang.
Investigation: Yan Li, Shaomin Yang, Xiaoguang Liu.
Methodology: Yan Li, Liang Jiang, Shaomin Yang, Xiaoguang Liu, Feng Wei, Hua Zhang, Zhongjun Liu.
Visualization: Liang Jiang, Zhongjun Liu.
Writing – original draft: Ben Wang, Siyu Zhou.
Writing – review and editing: Liang Jiang.
Footnotes
Abbreviations: AUC = area under the receiver operating characteristic curve, CI = confidence interval, CT = computed tomography, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio, ROC = receiver operating characteristic.
YL, BW, and SZ contributed equally to this study.
The study was approved by the ethics committee of Peking University Third Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
This study was funded by Peking University Third Hospital (Y71508-01). Author LJ has received grants from Peking University Third Hospital.
The authors have no conflicts of interest to disclose.
References
- [1].Boriani S, Biagini R, De Iure F, et al. Primary bone tumors of the spine: a survey of the evaluation and treatment at the Istituto Ortopedico Rizzoli. Orthopedics 1995;18:993–1000. [DOI] [PubMed] [Google Scholar]
- [2].Biagini R, Boriani S, De Iure F, et al. Vertebral tumors: differential diagnosis between primary and secondary neoplasms. La Chirurgia degli organi di movimento 1998;83:5–6. [PubMed] [Google Scholar]
- [3].Steinmetz MP, Mekhail A, Benzel EC. Management of metastatic tumors of the spine: strategies and operative indications. Neurosurg Focus 2001;11:e2. [DOI] [PubMed] [Google Scholar]
- [4].Sundaresan N, Boriani S, Rothman A, et al. Tumors of the osseous spine. J Neurooncol 2004;69:273–90. [DOI] [PubMed] [Google Scholar]
- [5].Khan SN, Donthineni R. Surgical management of metastatic spine tumors. Orthop Clin North Am 2006;37:99–104. [DOI] [PubMed] [Google Scholar]
- [6].Chi JH, Bydon A, Hsieh P, et al. Epidemiology and demographics for primary vertebral tumors. Neurosurg Clin North Am 2008;19:1–4. [DOI] [PubMed] [Google Scholar]
- [7].Lewis VO. What's new in musculoskeletal oncology. J Bone Jt Surg Am Vol 2009;91:1546–56. [DOI] [PubMed] [Google Scholar]
- [8].Dang L, Liu X, Dang G, et al. Primary tumors of the spine: a review of clinical features in 438 patients. J Neurooncol 2015;121:513–20. [DOI] [PubMed] [Google Scholar]
- [9].Boriani S, Saravanja D, Yamada Y, et al. Challenges of local recurrence and cure in low grade malignant tumors of the spine. Spine 2009;34(22 Suppl):S48–57. [DOI] [PubMed] [Google Scholar]
- [10].Schwab JH, Springfield DS, Raskin KA, et al. What's new in primary malignant musculoskeletal tumors. J Bone Jt Surg Am Vol 2013;95:2240–6. [DOI] [PubMed] [Google Scholar]
- [11].Liu X, Liu Z, Dang G. CT-guided percutaneous biopsy of the spine (352 cases review). Chin J Spine Spinal Cord 2004;2:18–21. [Google Scholar]
- [12].Donthineni R. Diagnosis and staging of spine tumors. Orthop Clin North Am 2009;40:1–7. [DOI] [PubMed] [Google Scholar]
- [13].Buyukbebeci O, Karakurum G, Tutar E, et al. Biopsy of vertebral tumour metastasis for diagnosing unknown primaries. J Orthop Surg (Hong Kong) 2010;18:361–3. [DOI] [PubMed] [Google Scholar]
- [14].Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493–503. [DOI] [PubMed] [Google Scholar]
- [15].Li Y, Jia H, Yu W, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer 2016;139:220–31. [DOI] [PubMed] [Google Scholar]
- [16].Kawahara T, Yokomizo Y, Ito Y, et al. Pretreatment neutrophil-to-lymphocyte ratio predicts the prognosis in patients with metastatic prostate cancer. BMC Cancer 2016;16:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Unal D, Eroglu C, Kurtul N, et al. Are neutrophil/lymphocyte and platelet/lymphocyte rates in patients with non-small cell lung cancer associated with treatment response and prognosis? Asian Pac J Cancer Prevent 2013;14:5237–42. [DOI] [PubMed] [Google Scholar]
- [18].Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer 2013;16:55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tan D, Fu Y, Su Q, et al. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Medicine 2016;95:e3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhou D, Zhang Y, Xu L, et al. A monocyte/granulocyte to lymphocyte ratio predicts survival in patients with hepatocellular carcinoma. Sci Reports 2015;5:15263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Deng Q, He B, Liu X, et al. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med 2015;13:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cho IR, Park JC, Park CH, et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer 2014;17:703–10. [DOI] [PubMed] [Google Scholar]
- [23].Nuhn P, Vaghasia AM, Goyal J, et al. Association of pretreatment neutrophil-to-lymphocyte ratio (NLR) and overall survival (OS) in patients with metastatic castration-resistant prostate cancer (mCRPC) treated with first-line docetaxel. BJU Int 2014;114:E11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li J, Li B, Zhou P, et al. Nomograms for prognostic factors of spinal giant cell tumor combining traditional clinical characteristics with inflammatory biomarkers after gross total resection. Oncotarget 2017;8:86934–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xia WK, Liu ZL, Shen D, et al. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J Surg Oncol 2016;14:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jiang Z, Shen K, Shen Y. Zhu Futang Practice of Pediatrics. 8 ed.Beijing: People's Medical Publishing House; 2015. [Google Scholar]
- [27].International Agency for Research on Cancer, Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed2013. [Google Scholar]
- [28].Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Laird BJ, Mcmillan DC, Fayers P, et al. The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. Oncologist 2013;18:1050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kostandinos S, Jaap K. Cancer inflammation and inflammatory biomarkers: can neutrophil, lymphocyte, and platelet counts represent the complexity of the immune system? Transpl Int 2014;27:28–31. [DOI] [PubMed] [Google Scholar]
- [31].Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol 2014;23:31–9. [DOI] [PubMed] [Google Scholar]
- [32].Elhag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol 1987;139:2406–13. [PubMed] [Google Scholar]
- [33].Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost 2011;9:237–49. [DOI] [PubMed] [Google Scholar]
- [34].Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137–48. [DOI] [PubMed] [Google Scholar]
- [35].Wu Q, Hu T, Zheng E, et al. Prognostic role of the lymphocyte-to-monocyte ratio in colorectal cancer: an up-to-date meta-analysis. Medicine 2017;96:e7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet (London, England) 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [37].Li Y, Liu X, Zhang J, et al. Prognostic role of elevated preoperative systemic inflammatory markers in localized soft tissue sarcoma. Cancer Biomark 2016;16:333–42. [DOI] [PubMed] [Google Scholar]
- [38].Rajeswaran G, Malik Q, Saifuddin A. Image-guided percutaneous spinal biopsy. Skeletal Radiol 2013;42:3–18. [DOI] [PubMed] [Google Scholar]
- [39].Kamei Y, Nishida J, Mimata Y, et al. Core needle percutaneous transpedicular vertebral body biopsy: a study of 128 cases. J Spinal Disord Tech 2015;28:E394–399. [DOI] [PubMed] [Google Scholar]


