Abstract
Background
Chronic lymphocytic leukaemia (CLL) accounts for 25% of all leukaemias and is the most common lymphoid malignancy in Western countries. Standard treatment includes mono‐ or poly‐chemotherapies. Nowadays, monoclonal antibodies are added, especially alemtuzumab and rituximab. However, the impact of these agents remains unclear, as there are hints of an increased risk of severe infections.
Objectives
To assess alemtuzumab compared with no further therapy, or with other anti‐leukaemic therapy in patients with CLL.
Search methods
We searched CENTRAL and MEDLINE (from January 1985 to November 2011), and EMBASE (from 1990 to 2009) as well as conference proceedings for randomised controlled trials (RCTs). Two review authors (KB, NS) independently screened search results.
Selection criteria
We included RCTs comparing alemtuzumab with no further therapy or comparing alemtuzumab with anti‐leukaemic therapy such as chemotherapy or monoclonal antibodies in patients with histologically‐confirmed B‐cell CLL. Both pretreated and chemotherapy‐naive patients were included.
Data collection and analysis
We used hazard ratios (HR) as an effect measure for overall survival (OS) and progression‐free survival (PFS) and risk ratios (RRs) for response rates, treatment‐related mortality (TRM) and adverse events. Two review authors independently extracted data and assessed the quality of trials.
Main results
Our search strategies led to 1542 potentially relevant references. Of these, we included five RCTs involving 845 patients. Overall, we judged the quality of the five trials as moderate. All trials were reported as randomised and open‐label studies. However, two trials were published as abstracts only, therefore, we were unable to assess the potential risk of bias for these trials in detail. Because of the small number of studies in each analysis (two), the quantification of heterogeneity was not reliable.
Two trials (N = 356) assessed the efficacy of alemtuzumab compared with no further therapy. One trial (N = 335), reported a statistically significant OS advantage for all patients receiving alemtuzumab (HR 0.65 (95% confidence interval (CI) 0.45 to 0.94; P = 0.021). However, no improvement was seen for the subgroup of patients in Rai stage I or II (HR 1.07; 95% CI 0.62 to 1.84; P = 0.82). In both trials, the complete response rate (CRR) (RR 2.61; 95% CI 1.26 to 5.42; P = 0.01) and PFS (HR 0.58; 95% CI 0.44 to 0.76; P < 0.0001) were statistically significantly increased under therapy with alemtuzumab. The potential heterogeneity seen in the forest plot could be due to the different study designs: One trial evaluated alemtuzumab additional to fludarabine as relapse therapy; the other trial examined alemtuzumab compared with no further therapy for consolidation after first remission.There was no statistically significant difference for TRM between both arms (RR 0.57; 95% CI 0.17 to 1.90; P = 0.36). A statistically significant higher rate of CMV reactivation (RR 10.52; 95% CI 1.42 to 77.68; P = 0.02) and infections (RR 1.32; 95% CI 1.01 to 1.74; P = 0.04) occurred in patients receiving alemtuzumab. Seven severe infections (64%) in the alemtuzumab arm in the GCLLSG CLL4B study led to premature closure.
Two trials (N = 177), evaluated alemtuzumab versus rituximab. Neither study reported OS or PFS. We could not detect a statistically significant difference for CRR (RR 0.85; 95% CI 0.67 to 1.08; P = 0.18) or TRM (RR 3.20; 95% CI 0.66 to 15.50; P = 0.15) between both arms. However, the CLL2007FMP trial was stopped early due to an increase in mortality in the alemtuzumab arm. More serious adverse events occurred in this arm (43% versus 22% (rituximab), P = 0.006).
One trial (N = 297), assessed the efficacy of alemtuzumab compared with chemotherapy (chlorambucil). For this trial, no HR is reported for OS. Median survival has not yet been reached, 84% of patients were alive in each arm at the data cut‐off or at the last follow‐up date (24.6 months). The TRM between arms shows no statistical significant difference (0.6% versus 2.0%; P = 0.34). Alemtuzumab statistically significantly improves PFS (HR 0.58; 95% CI 0.43 to 0.77; P = 0.0001), time to next treatment (23.3 compared with 14.7 months; P = 0.0001), ORR (83.2% versus 55.4%; P < 0.0001), CRR (24.2% versus 2.0%; P < 0.0001), and minimal residual disease rate (7.4% versus 0%; P = 0.0008) compared with chlorambucil. Statistically, significantly more asymptomatic (51.7% versus 7.4%) and symptomatic cytomegalovirus (CMV) infections (15.4% versus 0%) occurred in the patients treated with alemtuzumab.
Authors' conclusions
In summary, the currently available evidence suggests an OS, CRR and PFS benefit for alemtuzumab compared with no further therapy, but an increased risk for infections in general, CMV infections and CMV reactivations. The role of alemtuzumab versus rituximab still remains unclear, further trials with longer follow‐up and overall survival as primary endpoint are needed to evaluate the effects of both agents compared with each other. Alemtuzumab compared with chlorambucil seems to be favourable in terms of PFS, but a longer follow‐up period and trials with overall survival as primary endpoint are needed to determine whether this effect will translate into a survival advantage.
Keywords: Humans; Alemtuzumab; Antibodies, Monoclonal, Humanized; Antibodies, Monoclonal, Humanized/therapeutic use; Antibodies, Monoclonal, Murine‐Derived; Antibodies, Monoclonal, Murine‐Derived/therapeutic use; Antineoplastic Agents; Antineoplastic Agents/therapeutic use; Chlorambucil; Chlorambucil/therapeutic use; Leukemia, Lymphocytic, Chronic, B‐Cell; Leukemia, Lymphocytic, Chronic, B‐Cell/drug therapy; Leukemia, Lymphocytic, Chronic, B‐Cell/mortality; Randomized Controlled Trials as Topic; Rituximab; Vidarabine; Vidarabine/analogs & derivatives; Vidarabine/therapeutic use
Plain language summary
The role of the monoclonal antibody alemtuzumab for treatment of people with chronic lymphocytic leukaemia
Chronic lymphocytic leukaemia (CLL) is a cancer and accounts for 25% of all leukaemias. The disease is the most common cancer of the lymphatic system in Western countries and is characterised by a highly variable clinical course and prognosis. Some patients may have minimal or no symptoms for many years with a normal life expectancy, without requiring treatment. Others are symptomatic at diagnosis or early thereafter and can experience infectious and autoimmune complications, leading to a reduced lifespan. Standard treatment includes chemotherapy with one or more agents. Nowadays monoclonal antibodies are added, especially alemtuzumab and rituximab. However, the impact of these agents remains unclear, as there were hints for increased overall survival, but also risk for severe infections in non‐randomised trials. In this systematic review we summarised and analysed the evidence from randomised controlled trials (RCTs) on efficacy and safety of alemtuzumab in the treatment of CLL. We searched several important medical databases such as CENTRAL, MEDLINE and EMBASE and found five RCTs fulfilling our pre‐defined inclusion criteria. We included trials that compared alemtuzumab with no further therapy or with anti‐cancer therapy in newly‐diagnosed or relapsed patients with CLL. In total, 845 patients were treated within the five trials.
Two trials assessed whether alemtuzumab is favourable compared with no further therapy. One trial reported data on overall survival, showing a significant advantage for those patients receiving additional alemtuzumab. The time without progression was statistically significantly improved in both trials with alemtuzumab, but more patients had an infection, especially a virus infection (cytomegalovirus infection). Because of severe infections, one trial was closed prematurely.
Two trials evaluated alemtuzumab versus rituximab. Neither study reported data on survival or survival without a relapse of the disease. We found no statistically significant differences for response to therapy or for deaths during study treatment. One trial was stopped early due to an increase in mortality in the alemtuzumab arm.
In the fifth trial alemtuzumab was compared with chemotherapy (chlorambucil). In this trial no difference in survival could be detected until the last publication of the study. Alemtuzumab statistically significantly improves the survival without a relapse, the time to anti‐cancer treatment for relapse, and the response rate. Again, more infections occurred in the patients treated with alemtuzumab, especially infections with the cytomegalovirus that could lead to lung and retina infections.
In summary, the currently available evidence suggests an survival advantage for alemtuzumab compared with no further therapy, but an increased risk for infections in general and for cytomegalovirus.
Summary of findings
Summary of findings for the main comparison. Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded) for chronic lymphocytic leukaemia.
| Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded) for chronic lymphocytic leukaemia | ||||||
| Patient or population: patients with chronic lymphocytic leukaemia Settings: Intervention: Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded) | |||||
|
Overall survival (median 2 years) |
Moderate risk | HR 0.65 (0.45 to 0.94) | 335 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 250 per 1000 |
171 per 1000 (121 to 237) |
|||||
| Progression free survival (median 2 years) | Moderate risk | HR 0.61 (0.47 to 0.81) | 356 (2 studies) | ⊕⊕⊝⊝ low2,3 | ||
| 500 per 1000 |
345 per 1000 (278 to 430) |
|||||
| Treatment related mortality | Study population | RR 0.57 (0.17 to 1.9) | 356 (2 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 40 per 1000 | 23 per 1000 (7 to 75) | |||||
| Cytomegalovirus reactivation | Study population | RR 10.52 (1.42 to 77.68) | 350 (2 studies) | ⊕⊕⊕⊕ high2,4 | ||
| 10 per 1000 |
105 per 1000 (14 to 777) |
|||||
| Complete response rate | Study population | RR 2.61 (1.26 to 5.42) | 356 (2 studies) | ⊕⊕⊝⊝ low2,3 | ||
| 51 per 1000 | 133 per 1000 (64 to 276) | |||||
| Infections (all grades) | Study population | RR 1.32 (1.01 to 1.74) | 356 (2 studies) | ⊕⊕⊝⊝ low2,3 | ||
| 331 per 1000 | 437 per 1000 (335 to 577) | |||||
| Serious adverse events | Study population | RR 1.34 (0.95 to 1.89) | 350 (2 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 234 per 1000 | 314 per 1000 (223 to 443) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1One trial only
2 One trial stopped early due to high incidence of CMV reactivation in alemtuzumab arm; two of 23 patients randomised refused initiation of study treatment after randomisation and were excluded from analysis (no ITT analysis) 3 Heterogeneity between trials
4 Large effect
Summary of findings 2. Anti‐leukaemic therapy with alemtuzumab versus anti‐leukaemic therapy without alemtuzumab (anti‐leukaemic therapy not identical in both groups, confounded) for chronic lymphocytic leukaemia.
| Anti‐leukaemic therapy with alemtuzumab versus anti‐leukaemic therapy without alemtuzumab (anti‐leukaemic therapy not identical in both groups, confounded) for chronic lymphocytic leukaemia | ||||||
| Patient or population: patients with chronic lymphocytic leukaemia Settings: Intervention: Anti‐leukaemic therapy with alemtuzumab versus anti‐leukaemic therapy without alemtuzumab (anti‐leukaemic therapy not identical in both groups, confounded) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Anti‐leukaemic therapy with alemtuzumab versus anti‐leukaemic therapy without alemtuzumab (anti‐leukaemic therapy not identical in both groups, confounded) | |||||
| Overall survival not reported | See comment | See comment | Not estimable | ‐ | See comment | Neither study provided data with regard to this outcome. |
|
Progression free survival not reported |
See comment | See comment | Not estimable | ‐ | See comment | Neither study provided data with regard to this outcome. |
| Treament related mortality | Study population | RR 3.2 (0.66 to 15.5) | 177 (2 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 22 per 1000 | 70 per 1000 (15 to 341) | |||||
| Complete response rate | Study population | RR 0.85 (0.67 to 1.08) | 170 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 655 per 1000 | 557 per 1000 (439 to 707) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One trial stopped prematurely due to an increase in mortality in the alemtuzumab arm.
2 Few events were observed for this outcome, leading to a wide confidence intervals.
Background
Description of the condition
Chronic lymphocytic leukaemia (CLL) accounts for 25% of all leukaemias and is the most common lymphoid malignancy in Western countries (Chiorazzi 2005). The disease is characterised by a highly variable clinical course and prognosis. Some patients may have minimal or no symptoms for many years with a normal life expectancy, without requiring treatment. Others are symptomatic at diagnosis or early thereafter. They experience infectious or autoimmune complications and may die of drug‐resistant disease much earlier than the normal life expectancy.
The extent of the disease is reflected by enlargement of lymph nodes, liver, and spleen; a raised lymphocyte count in the blood; and a degree of impairment of normal haematopoiesis. These variables can be used to define the different stages of the disease. The two most widely used staging systems, proposed by Rai et al and Binet and co‐workers, discriminate between early (Rai 0; Binet A), intermediate (Rai I,II; Binet B), and advanced (Rai III/IV; Binet C) disease with substantial differences in clinical course and long‐term survival. However, these clinical staging systems are often of limited prognostic value at diagnosis, when most patients are in the early stages of the disease (Binet 1981; Hallek 2008; Rai 1975). Recently, other prognostic factors have been identified which distinguish better between more and less active forms of the disease. In particular, patients with a 17p deletion have an aggressive form of the disease with a median survival of less than one year (Dohner 2000).
Most patients with CLL are treated when they have an advanced stage of the disease, when they are symptomatic or have haematopoietic insufficiency. Standard treatment options include monotherapy with chlorambucil, bendamustine, or purine analogues (fludarabine, pentostatine); polychemotherapies with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP); cyclophosphamide, vincristine, with prednisolone (COP); or fludarabine with cyclophosphamide (FC). During the last few years, the addition of monoclonal antibodies (moAbs) to chemotherapy or antibody monotherapy has moved into the focus of interest.
While fludarabine leads to higher response rates and longer progression‐free survival (PFS) compared with other monotherapies, CHOP, or COP do not improve the overall survival (OS), as shown in a Cochrane Review (Steurer 2006). The same is true for the combination of fludarabine and cyclophosphamide compared with fludarabine alone in randomised trials (Eichhorst 2006; Flinn 2007). So far, there are no randomised data showing an impact on OS for the various treatment options. On the other hand, patients with CLL are at increased risk of infections and infectious complications, including death. This may be related to the disease itself, the consequences of therapy, or both. Indeed, infections are more pronounced with treatments leading to longer PFS (for example fludarabine, or the combination of fludarabine with cyclophosphamide) (Hallek 2008).
Monoclonal antibodies against surface proteins expressed in CLL cells may allow a more targeted therapy for CLL. Examples are alemtuzumab, directed against CD52; rituximab (anti‐CD20); ofatumumab (anti‐CD20); and lumiliximab (anti‐CD23). Both alemtuzumab and rituximab have shown improved PFS compared with treatment without antibodies (Hallek 2008a; Hillmen 2007). In a retrospective analysis comparing FC with FC‐rituximab (FCR), Wierda et al showed a possible benefit on OS (Wierda 2006). A benefit for OS was also shown for relapsed or refractory patients with minimal residual disease (MRD) negativity after alemtuzumab treatment (Moreton 2005). This review is part of a series of reviews examining the role of monoclonal antibodies in CLL (for the role of rituximab in patients with CLL see (Weingart 2009)).
Description of the intervention
In a first small phase II trial, treatment of 93 fludarabine refractory patients with alemtuzumab resulted in an overall response rate (ORR) of 33% and a median OS of 16 months (32 months OS for patients who responded) (Keating 2002). Results seemed to improve when combining alemtuzumab with chemotherapy in other phase II trials for relapsed and refractory patients (Elter 2005; Wierda 2005).
A recently published randomised controlled trial (RCT) comparing alemtuzumab with chlorambucil in the first‐line therapy of patients with CLL showed a significantly improved PFS (hazard ratio (HR) log rank 0.58 confidence interval (CI): 0.43 to 0.77) (Hillmen 2007). The OS after a median follow‐up of 24.6 months showed no difference, with 84% of participants surviving in each arm. Patients receiving chlorambucil did not have cytomegalovirus (CMV) infections with symptoms but 15.6% of patients receiving alemtuzumab had symptomatic PCR‐positive CMV infections. Based on similar results, screening for asymptomatic CMV infections and prophylactic treatment of CMV has been advocated (Thursky 2006).
Patients in complete or partial remission after first‐line chemotherapy with either fludarabine or fludarabine plus cyclophosphamide were randomised to alemtuzumab or no intervention. At 21.4 months median follow‐up, patients receiving alemtuzumab showed a significant longer PFS (no progression versus 24.7 months, P = 0.036). However, this trial was stopped early due to severe toxicity and infectious complications in the alemtuzumab group (Wendtner 2004). Further randomised studies evaluating the effectiveness of alemtuzumab alone or in combination with chemotherapy are currently underway (see Ongoing studies).
How the intervention might work
The new age of cancer therapy started in 1975, when hybridoma technology led to the development of monoclonal antibodies. These antibodies, applied as a single‐agent or combination therapy, attempt to improve anti‐tumour activities or decrease the treatment‐associated toxicity on the basis of a targeted therapy. One of these antibodies is alemtuzumab (drug name e.g. Campath, MabCampath), a humanised antibody specific for CD52, a surface protein present on CLL B‐cells as well as normal B and T‐cells (Wierda 2005). Alemtuzumab was approved by the FDA for CLL in 2001 and is also successfully used in patients with multiple sclerosis (Coles 2011).
Why it is important to do this review
Based on published trials, alemtuzumab may be an effective treatment option for patients with CLL with possible benefits on OS (Moreton 2005). On the other hand, there are serious side effects and the non‐randomised design of most of the trials may introduce biases that can overestimate the benefit of these new therapeutic agents (Flynn 2007). At this stage, no systematic review or meta‐analysis of alemtuzumab in patients with CLL is available. We are aiming to obtain more evidence regarding the clinical benefit (OS, PFS, response rate) and the therapy‐related risks (treatment‐related mortality (TRM), adverse events), by systematically analysing the reliability and validity of the data and by considering only RCTs for our review. If this is reasonable, we will summarise these results in a meta‐analysis and re‐evaluate the use of alemtuzumab in the treatment of CLL. Our review is intended to contribute to decision support for effective treatment strategies with the best balance between benefits and harms for the individual patient.
Objectives
The objectives of this review are to assess and summarise the evidence on efficacy and safety of alemtuzumab in the treatment of CLL, both in newly diagnosed and relapsed patients.
Methods
Criteria for considering studies for this review
Types of studies
We only considered RCTS. We included both full‐text and abstract publications, if sufficient information was available on study design, characteristics of participants, interventions and outcomes.
Types of participants
We included trials on patients with histologically confirmed B‐cell CLL. We included trials with both pretreated and chemotherapy‐naive patients. If we had found trials with mixed populations, i.e. patients with different haematological malignancies, we would only have used the data from the CLL subgroups. If subgroup data for patients with CLL had not have been provided (after contacting the authors of the trial), we would have excluded the trial if less than 80% of patients had CLL.
Types of interventions
We included RCTs evaluating alemtuzumab alone or in combination with chemotherapy as primary treatment, maintenance treatment, or treatment in refractory patients. We considered different treatment approaches for CLL considered as the control group, including conventional therapy such as fludarabine or chlorambucil monotherapy, fludarabine in combination with other chemotherapeutic agents, or another antibody therapy.
We considered trials of alemtuzumab in designs where the only difference between the treatment and control arms is the addition of alemtuzumab and in designs where there are additional differences between the treatment arms. We also considered dose comparison studies of alemtuzumab.
We examined the following types of comparisons:
anti‐leukaemic therapy with alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups);
anti‐leukaemic therapy with alemtuzumab versus anti‐leukaemic therapy without alemtuzumab (anti‐leukaemic therapy not identical in both groups);
alemtuzumab versus anti‐leukaemic therapy.
We would have examined different dosages or time schedules of alemtuzumab, but we did not identify any trial fulfilling these criteria.
Types of outcome measures
Primary outcomes
Overall survival (OS); defined as the time interval from random treatment assignment/entry into the study to death from any cause or to last follow‐up.
Secondary outcomes
We analysed the following outcomes as secondary outcomes:
progression‐free survival (PFS);
time to next treatment;
treatment‐related mortality (TRM);
complete response rate (CRR);
overall response rate (ORR);
minimal residual disease (MRD);
adverse events;
number of patients discontinuing the study because of drug‐related adverse events.
Search methods for identification of studies
Electronic searches
We adapted the search strategies suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). No language restriction was applied to reduce the language bias.
Bibliographic databases
Cochrane central register of controlled trials (CENTRAL) (The Cochrane Library 2011, Issue 11), search strategy see Appendix 1;
Ovid MEDLINE (1990 to 18.11.2011), search strategy see Appendix 2;
Ovid EMBASE (1990 to 20.03.2009), search strategy see Appendix 3.
Conference proceedings
We searched conference proceedings of relevant conferences of the following societies for the years they were not included in CENTRAL:
American Society of Clinical Oncology (ASCO) (2009; 2010);
American Society of Haematology (ASH) (2009; 2010);
European Haematology Association (EHA) (2009; 2010);
European Society of Medical Oncology (ESMO) (2010).
Electronic search in databases of ongoing trials
We searched the metaRegister of Controlled Trials (mRCT) to identify ongoing trials (www.clinicaltrials.gov).
Searching other resources
We handsearched references:
references of all identified trials, relevant review articles;
current treatment guidelines (www.g‐i‐n.net).
Institutions
We searched the websites of relevant institutions, agencies (for example, the Food and Drug Administration (FDA (www.fda.gov)), organisations (CLL study groups), and the pharmaceutical company (Genzyme Oncology) for completed and ongoing studies.
Personal contacts
We contacted the authors of relevant studies for unpublished material.
Data collection and analysis
Selection of studies
Two review authors (NS, KB) independently screened the results of the search strategies for eligibility for this review by reading relevant abstracts. In case of disagreement, we obtained the full‐text publication (Higgins 2011). If no consensus had been reached, we would have asked a third review author to give his or her opinion, but this procedure was not necessary.
We documented the study selection process in a flow chart as recommended in the PRISMA statement (Moher 2009) showing the total numbers of retrieved references and the numbers of included and excluded studies.
Data extraction and management
Two review authors (NS, KB) independently extracted the data according to the guidelines proposed by The Cochrane Collaboration (Higgins 2011). We would have contacted the authors of individual studies for additional information, but it was not required. We used a standardised data extraction form containing the following items:
general information: study ID; author; title; journal; publication date; citation and contact details of primary or corresponding authors; sources of funding;
study characteristics: design; objectives and duration of the study; source of participants; number of participating centres; inclusion and exclusion criteria; sample size; treatment allocation; comparability of groups; subgroup analysis; statistical methods; power calculations; compliance with assigned treatment; length of follow‐up;
participant characteristics: age; sex; ethnicity; setting; number of participants recruited/ randomised/ evaluated; additional diagnoses; stage of the disease; numbers of participants lost to follow‐up; drop outs (percentage in each arm) with reasons; protocol violations; previous treatments; prognostic factors;
interventions: setting; dose and duration of alemtuzumab; type, dosage and duration of chemotherapy (number of cycles); administration route; supportive treatment; compliance to interventions; additional interventions given; any difference between interventions;
outcomes: OS; PFS; response rate; time to next treatment; TRM; minimal residual disease rate; adverse events; number of patients discontinuing the study because of drug‐related adverse events; number of patients evaluated for primary outcomes; number of patients evaluated for secondary outcomes; length of follow‐up for survival endpoints;, and definitions for the outcomes.
We used both full‐text versions and abstracts including additional information (for example slides) of eligible studies to retrieve the data. We extracted trials reported in more than one publication on one form only. Where these sources did not provide sufficient information, we had planned to contact the authors for additional details, however, this was not necessary.
Assessment of risk of bias in included studies
To assess quality and risk of bias, we used a questionnaire (validity assessment form) containing the items as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a):
sequence generation;
allocation concealment;
blinding (participants, personnel, outcome assessors);
incomplete outcome data;
selective outcome reporting;
other sources of bias.
For every criterion, we made a judgement using one of three categories:
'Low risk': if the criterion is adequately fulfilled in the study, i.e. the study is at a low risk of bias for the given criterion;
'High risk': if the criterion is not fulfilled in the study, i.e. the study is at high risk of bias for the given criterion;
'Unclear': if the study report does not provide sufficient information to allow for a judgement of 'Yes' or 'No' or if the risk of bias is unknown for one of the criteria listed above.
Measures of treatment effect
We estimated treatment effect measures of individual studies as relative effect measures (risk ratio (RR)) with 95% confidence intervals (CI) for dichotomous data. For survival data, we estimated treatment effects of individual studies as hazards ratios (HR) using the methods described by Parmar (Parmar 1998) and Tierney (Tierney 2007).
Dealing with missing data
As suggested in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), there are many potential sources of missing data which have to be taken into account: at study level, at outcome level, and at summary data level. Firstly, it is important to distinguish between "missing at random" and "not missing at random".
We contacted the original investigator to request missing data. We performed sensitivity analysis to assess how sensitive results were to reasonable changes in the assumptions that we made. We addressed the potential impact of missing data on the finding of the review in the discussion.
Assessment of heterogeneity
Because of the small number of studies in each analysis (two), the quantification of heterogeneity is not reliable, since the confidence interval is huge. In meta‐analyses with more trials, we would have assessed heterogeneity of treatment effects between trials using a Chi² test with a significance level at P < 0.1. In that case, we would have used the I² statistic to quantify possible heterogeneity (I² > 30% moderate heterogeneity, I² > 75% considerable heterogeneity) (Deeks 2011). We explored potential causes of heterogeneity by sensitivity and subgroup analyses where possible.
Assessment of reporting biases
In a meta‐analysis with more than 10 trials, we would have explored potential reporting bias by generating a funnel plot and statistically testing this by conducting a linear regression test (Sterne 2011). A P value less than 0.1 would have been considered significant for this test. However, we only included five trials so this test was not done.
Data synthesis
We performed analyses according to the recommendations of Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We used aggregated data for analysis. For statistical analysis, we entered data into the Cochrane statistical package Review Manager (RevMan) 5.1. One review author (NS) entered data into RevMan software and a second review author (KB) checked it for accuracy. We performed meta‐analyses using a fixed‐effect model (generic inverse variance method for survival data outcomes and Mantel‐Haenszel method for dichotomous data outcomes). With more included trials, we would have used the random‐effects model in terms of sensitivity analyses.
If appropriate, we calculated the number needed to treat to benefit and the number needed to treat to harm.
We used the software GRADEpro 3.2 to create 'Summary of Finding' tables as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011).
Subgroup analysis and investigation of heterogeneity
Because of the small number of studies in each analysis and missing subgroup data, it was not possible to explore heterogeneity in full detail. We would have taken the following parameters into consideration for subgroup analyses as there is some evidence that these parameters could cause heterogeneity, but the study authors did not provide subgroup data:
age (e.g. adults < 50 years versus adults ≥ 50 years);
stage of disease;
influence of prognostic factors; e.g. 11q‐ or 17p‐deletion.
We evaluated the following parameters in subgroup analyses:
different treatment approaches (e.g. combination with chemotherapy or not);
different alemtuzumab approaches (e.g. primary therapy, maintenance or relapse).
Sensitivity analysis
We assessed robustness of the overall results by sensitivity analysis with respect to the quality and design of trials. Due to the low number of trials included in each analysis (two), we evaluated the influence of full‐text versus abstract publication only.
Results
Description of studies
Results of the search
We identified 1542 potentially relevant references through database searches and handsearching. Of these, 1502 were excluded at the initial stage of screening because they did not fulfil our pre‐defined inclusion criteria or were duplicates. The remaining 40 publications were retrieved as full‐text publications or abstract publications for detailed evaluation. Of these 40 publications, we excluded 10 publications and finally 30 publications (five trials) with 845 patients were formally included in this systematic review. We included two trials in the main meta‐analyses of this review. The overall number of references screened, identified, selected, excluded and included are documented according to the PRISMA flow diagram (Figure 1).
1.
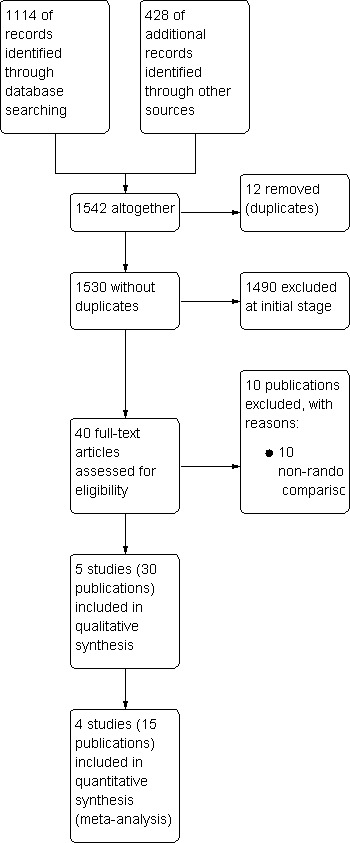
Flow diagram.
One guideline was identified but with no additional RCT (Fraser 2006).
Included studies
Five trials in 30 publications including a total of 845 patients (range 12 to 335 patients per trial), fulfilled the inclusion criteria (CAM 307; CAM 314; CLL2007FMP; GCLLSG CLL4B; Gribben 2005).
The characteristics of included trials are summarised in Characteristics of included studies.
For two trials no dates on trial recruitment were provided. One trial recruited from 2001 to 2004 (CAM 307), one from July 2004 to October 2008 (CAM 314), and the other trial from 2007 to 2009 (CLL2007FMP). Two review authors extracted data from full‐text publications for three trials (CAM 307; CAM 314; GCLLSG CLL4B) and from abstract publications for two trials (CLL2007FMP; Gribben 2005).
Design
All five included trials were two‐armed RCTs.
Sample sizes
The smallest trial (Gribben 2005) randomised 12 patients and the largest trial 335 patients (CAM 314).
Location
Four included trials were conducted in Europe and the US (CAM 307; CAM 314; CLL2007FMP; GCLLSG CLL4B); the other trial (Gribben 2005), did not report where the trial took place.
Participants
A total of 832 male and female patients with histologically‐proven CLL were randomised. In two trials alemtuzumab was evaluated in patients receiving first‐line therapy (CAM 307; CLL2007FMP), in one trial as maintenance therapy after response to primary therapy (GCLLSG CLL4B) and in two trials in relapsed or refractory patients (CAM 314; Gribben 2005).
Interventions
In two trials, alemtuzumab was evaluated versus no further therapy and observation (CAM 314; GCLLSG CLL4B). Two trials assessed the role of alemtuzumab compared with rituximab (CLL2007FMP; Gribben 2005) and one trial evaluated alemtuzumab compared with chemotherapy (chlorambucil) (CAM 307).
Outcomes
Primary outcome measure
Overall survival was reported in one trial only (CAM 314), although two further trials were reported as full‐texts (CAM 307; GCLLSG CLL4B).
Secondary outcome measures
Three trials reported PFS (CAM 307; CAM 314; GCLLSG CLL4B). Response rate was analysed in all five trials, minimal residual disease was evaluated in three trials (CAM 307; CLL2007FMP; GCLLSG CLL4B). Four trials mentioned TRM (CAM 307; CAM 314; GCLLSG CLL4B; CLL2007FMP), and all trials reported adverse events.
Conflict of interest
One trial did not provide conflict of interest statements (abstract publication) Gribben 2005). In one trial, the authors indicated no potential conflict of interest (CLL2007FMP). One trial was supported by a research grant of Schering AG, Berlin and MedacSchering Onkologie, Germany (GCLLSG CLL4B). All authors of CAM 307 are either employees or consultants for Genzyme Corp or have received research grants from Genzyme Corp. One author is a consultant or has a advisory role for Bayer Schering Pharma. Most authors of (CAM 314) received honorariums as members of a board of directors or advisory committees for Genzyme. Two authors of this trial are employees of Genzyme.
Excluded studies
We excluded 10 trials of the retrieved full‐text publications, because they were not RCTs (Bolli 2004; Byrd 2009; Elter 2009; Faderl 2010; Hale 2004; Karlsson 2006; Kennedy 2000; Lin 2010; Osterborg 1996; Pettitt 2006).
Risk of bias in included studies
Overall, the quality of included trials was moderate. Two included trials were published as abstracts only, therefore, we were not able to assess the potential risk of bias for these trials in detail. For more information see the 'Risk of bias' tables of included trials and for an overview of the results, please see Figure 2 and Figure 3.
2.
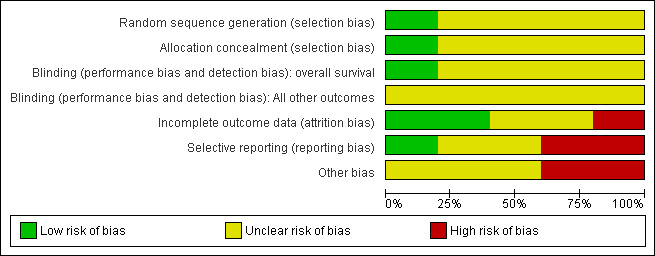
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
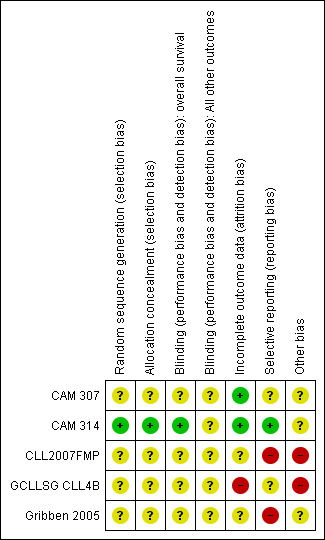
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In one trial, the random sequence generation (minimisation method) and the allocation concealment were adequate (CAM 314); no information was available for the other trials.
Blinding
Patients and physicians were not blinded in the five included trials; no information is given for blinding of the outcome assessor or statistician.
Primary outcome (overall survival)
Although the trial evaluating OS did not report blinding of the outcome assessor, we judged the potential risk of bias for this outcome as "low" in this trial, as death is an endpoint not susceptible to be biased by the outcome assessor (CAM 314).
Secondary outcomes
As blinding of the outcome assessors is considered important for this review, we judged all trials as "unclear" for the question of blinding.
Incomplete outcome data
Two of the three trials published as full‐texts described missing outcome data in detail and stated that they performed analyses according to the intention‐to‐treat‐principle (CAM 307; CAM 314), we therefore, judged risk of attrition bias for this trial as "low". In the other trial published as full‐text, two of 23 patients refused treatment after randomisation and were not included in the final analysis (GCLLSG CLL4B). We judged risk of incomplete outcome data for this trial as "high".
There were no obvious missing data among the two trials published as abstracts only, but no detailed information on statistical methods and patients analysed were given, therefore, we judged the risk of attrition bias for these trials as "unclear" (CLL2007FMP; Gribben 2005).
Selective reporting
For one trial, there is no study protocol in http://www.controlled‐trials.com/mrct/ available, therefore, we were not able to judge on the potential risk of reporting bias (GCLLSG CLL4B). For one study, a protocol is registered, but no information for pre‐planned outcomes is given (CAM 307). For those two studies, we judged the risk of bias as "unclear". Most of the patient‐important pre‐planned outcomes and the primary endpoint are reported in the full‐text publication of CAM 314, however, quality of life is not reported. Although this outcome is not reported, we judged the risk of bias for this trial as "low", as quality of life often is reported in separate publications. The other two studies were published as abstracts and only a few outcomes of the pre‐planned outcomes were reported. Therefore, we judged the risk of reporting bias for these three trials as "high".
Other potential sources of bias
Two trials were stopped prematurely due to an increased incidence of severe infections or an increase in mortality in the alemtuzumab arm (CLL2007FMP; GCLLSG CLL4B). Both trials were judged as having a potential "high risk" of bias. One trial recruited more patients than planned (335 instead of 300) without a clear rationale (CAM 314). The risk of bias for this trial was judged as "unclear".
Effects of interventions
Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups)
Two RCTs (N = 356) evaluated the efficacy and safety of alemtuzumab in an unfounded design (CAM 314; GCLLSG CLL4B).
Overall survival (OS)
Only one of the two trials provided HR for the primary endpoint OS (CAM 314). The authors of CAM 314 reported a statistically significant Improvement in OS for patients receiving fludarabine plus alemtuzumab compared with fludarabine only (HR 0.65 (95% CI 0.45 to 0.94, P = 0.021)) (CAM 314). No improvement in OS was seen in the subgroup of patients with Rai stage I or II (HR 1.07; 95% CI 0.62 to 1.84; P = 0.82).
The authors of the trial by the German CLL study group (GCLLSG) reported that 19 patients were alive at last follow‐up (median 48 months), without providing HRs or survival curves (GCLLSG CLL4B). Two patients died because of progressive disease (one patient in each arm).
Progression‐free survival (PFS)
Both trials (N = 356) reported a statistically significant improvement for PFS through the addition of alemtuzumab (HR 0.58; 95% CI 0.44 to 0.76; P < 0.0001). There could be some heterogeneity, visible in the forest plot between trials, probably due to the different study designs: CAM 314 evaluated alemtuzumab additional to fludarabine as relapse therapy. GCLLSG CLL4B examined alemtuzumab compared with no further consolidation after the first remission.
Nevertheless, the statistically significant effect is visible in both single trials: In CAM 314, additional alemtuzumab led to improved PFS (HR 0.61; 95% CI 0.47 to 0.81; P = 0.0003); an even more pronounced effect is seen in GCLLSG CLL4B (HR 0.17; 95% CI 0.05 to 0.60; P = 0.006).
Time to next treatment
Neither study reported on this outcome.
Treatment‐related mortality (TRM)
Although less treatment‐related mortality occurred in the alemtuzumab arm, there is no statistically significant difference for TRM in these two studies between both arms (N = 356; RR 0.57; 95% CI 0.17 to 1.90; P = 0.36).
Engert et al reported TRM, defined as death occurring on therapy or within 30 days after the last dose. Four of 168 patients (2%) died on fludarabine plus alemtuzumab arm versus seven of 167 (4%) on fludarabine‐only arm. For the subgroup of patients in the advanced stage, two patients (3%) died in the additional alemtuzumab arm versus five patients (8%) in the fludarabine‐only arm (CAM 314).
Wendtner et al reported that no patient died during treatment (altogether two patients died during the follow‐up phase, both because of progressive disease) (GCLLSG CLL4B).
Overall response rate (ORR)
Although the overall response rate is increased in patients receiving alemtuzumab, there is no statistically significant difference for the ORR between patients who did, or did not, receive alemtuzumab (RR 1.10; 95% CI 0.99 to 1.23; P = 0.09).
The relative chance for overall response in CAM 314 (relapse therapy; alemtuzumab combined with fludarabine) is RR = 1.08 (95% CI 0.97 to 1.21; P = 0.18).
The relative chance in GCLLSG CLL4B (consolidation after first remission; alemtuzumab not combined with other chemotherapy) is RR = 1.41 (95% CI 0.92 to 2.14; P = 0.11). However, there is a difference in response to pretreatment prior to randomisation and alemtuzumab therapy: in the alemtuzumab arm there were: one complete response (CR) and 10 partial responses (PR) compared with two CR, five PR, and three nodular PR in the arm without further therapy.
Complete response rate (CRR)
The CRR is statistically significantly higher in the alemtuzumab arm (RR 2.61; 95% CI 1.26 to 5.42; P = 0.01). The potential heterogeneity seen in the forest plot could be explained by the baseline imbalance in the GCLLSG CLL4B study: prior to randomisation to alemtuzumab there were one CR and 10 PR in the alemtuzumab arm versus two CR, five PR and three nodular PR in the arm without any further treatment.
In CAM 314 (relapse therapy; alemtuzumab combined with fludarabine), the relative chance for CR is statistically significantly higher in the alemtuzumab arm (RR 2.98; 95% CI 1.30 to 6.83; P = 0.01).
In GCLLSG CLL4B (consolidation after first‐remission; alemtuzumab not combined with other chemotherapy) there is no statisticallysignificant effect between both arms (RR 1.36; 95% CI 0.28 to 6.56; P = 0.70).
Minimal residual disease (MRD)
CAM 314 did not report any information regarding MRD.
GCLLSG CLL4B reported data for 9 of 21 participants (six in the alemtuzumab and three in the observation arm). Due to the high proportion of missing data, we did not calculate a MRD rate.
Adverse events
CMV reactivation
Both studies reported on various adverse events. A statistically significant higher rate of CMV reactivation and symptomatic CMV infection occurred in patients receiving alemtuzumab (RR 10.52; 95% CI 1.42 to 77.68; P = 0.02).
In both single studies, more CMV infections occurred in the alemtuzumab arm, but this effect is is not statistically significantly increased. In CAM 314 (relapse therapy; alemtuzumab combined with fludarabine) the relative risk of symptomatic CMV infection is RR = 9.05 (95% CI 0.49 to 166.84; P = 0.14).
In GCLLSG CLL4B (consolidation after first‐remission therapy; alemtuzumab not combined with other chemotherapy) the effect is RR = 11.92 (95% CI 0.76 to 187.84; P = 0.08).
All grade infections
Statistically significant more infections occurred in patients receiving alemtuzumab (RR 1.32; 95% CI 1.01 to 1.74; P = 0.04). However, there could be heterogeneity between trials, visible in the forest plot.
In CAM 314 (relapse therapy; alemtuzumab combined with fludarabine), the relative risk for all grade infections is not statistically significantly different between groups (RR 1.16; 95% CI 0.88 to 1.53; P = 0.29).
In GCLLSG CLL4B (consolidation after first‐remission therapy; alemtuzumab not combined with other chemotherapy), we found a statistically significant higher infection rate in the alemtuzumab arm (RR 19.25; 95% CI 1.27 to 291.20; P = 0.03). The incidence of seven severe infections in the alemtuzumab arm led to premature closure of the trial.
Haematological toxicity
Slightly less grade 3 or 4 haematological toxicities occurred in the alemtuzumab arm, but these differences were not statistically significant (anaemia: (RR 0.63; 95% CI 0.33 to 1.20; P = 0.16); neutropenia (RR 1.25; 95% CI 0.97 to 1.61); P = 0.09); thrombocytopenia (RR 1.05; 95% CI 0.58 to 1.89; P = 0.88).
Elter et al reported that adverse events occurring in >10% in the additional alemtuzumab arm relative to fludarabine‐only arm include pyrexia, leucopenia, chills, lymphopenia, urticaria, infusion‐related reactions, and rash (CAM 314).
Serious adverse events (SAEs)
More serious adverse events were found in the alemtuzumab arm, but there is no statistically significant difference between patients who received additional alemtuzumab and those who did not (RR 1.34; 95% CI 0.95 to 1.89; P = 0.09).
Serious AEs occurred in 33% of participants in the additional alemtuzumab and 25% in the fludarabine arm (CAM 314).
Number of patients discontinuing the study because of drug‐related adverse events
In CAM 314, 37 patients (23%) in the alemtuzumab arm and 32 patients (19%) in the fludarabine‐only group had to discontinue the study because of adverse events. However, they were assessed for the study outcomes and their data included in the analyses.
GCLLSG CLL4B reported no data on how many patients in both arms discontinued the study because of drug‐related adverse events. However, seven patients (63.6%) in the alemtuzumab‐arm developed acute severe infection and the trial had to be stopped prematurely.
Anti‐leukaemic therapy with alemtuzumab versus anti‐leukaemic therapy without alemtuzumab (anti‐leukaemic therapy not identical in both groups)
Two RCTs (N = 177) evaluated the role of anti‐leukaemic therapy with alemtuzumab compared to another anti‐leukaemic therapy without alemtuzumab (CLL2007FMP; Gribben 2005). In both studies, patients were randomised to receive additional alemtuzumab or additional rituximab.
Overall survival (OS)
Neither study provided data with regard to this outcome.
Progression‐free survival (PFS)
Neither study reported PFS data.
Time to next treatment
In both studies there were no data provided for time to next treatment.
Treatment related mortality (TRM)
Both trials reported on TRM (N = 177 patients). Treatment related mortality was increased in the alemtuzumab arm compared to the rituximab arm and one trial (CLL2007FMP) was stopped prematurely due to an increase in mortality in the alemtuzumab arm. Although no statistically significant difference was detectable between patients receiving additional alemtuzumab compared with those receiving additional rituximab (RR 3.20; 95% CI 0.66 to 15.50; P = 0.15), a RR 0f 3.20 could b of concern if maintained on more robust data. The potential heterogeneity seen in the forest plot between trials could be due to different patients characteristics:
In CLL2007FMP, patients received alemtuzumab or rituximab for first‐line treatment (RR 9.11; 95% CI 0.50 to 166.53; P = 0.14).
Gribben 2005 reported results for relapsed disease (RR 1.00; 95% CI 0.13 to 8.00; P = 1.00).
Overall response rate (ORR)
Both trials reported on ORR (N = 170 patients). No statistically significant difference could be found for the ORR (RR 0.95; 95% CI 0.85 to 1.07; P = 0.43).
In CLL2007FMP (first‐line treatment), the chance for OR was RR = 0.93 (95% CI 0.83 to 1.04; P = 0.21). In Gribben 2005 (relapse therapy) RR = 1.75 (95% CI 0.63 to 4.88; P = 0.28).
Complete response rate (CRR)
There was no statistically significant difference in terms of CRR between the patients receiving additional alemtuzumab and those receiving additional rituximab (RR 0.85; 95% CI 0.67 to 1.08; P = 0.18).
In CLL2007FMP (first‐line treatment), the chance for CR was RR = 0.81 (95% CI 0.64 to 1.03; P = 0.09). In Gribben 2005 (relapse therapy) RR = 3.50 (95% CI 0.45 to 27.52; P = 0.23).
Minimal residual disease (MRD)
CLL2007FMP reported that at nine months, 21 patients (26%) in the alemtuzumab arm and 36 patients (45%) in the rituximab arm were MDR‐negative.
Gribben 2005 did not report any data for this outcome.
Adverse events
In the Gribben 2005 trial, two CMV reactivations (50%) were reported in the additional alemtuzumab arm. The number of CMV reactivations for patients receiving additional rituximab was not reported.
CLL2007FMP provided data for all grade 3/4 adverse events (72 patients (87%) in the alemtuzumab arm versus 75 patients (90%) in the rituximab arm, P = 0.76). There was no statistically significant difference for grade 3/4 neutropenia (65 patients (79%) in the alemtuzumab arm versus 62 patients (75%), P = 0.49). However, SAEs were statistically significantly more frequent in the alemtuzumab arm (in 35 patients (43%) compared with 18 patients (22%) in the rituximab arm, P = 0.006). Additionally, serious febrile neutropenia was statistically significantly more frequent in the alemtuzumab arm (27 patients (33%) versus 13 patients (16%), P = 0.01).
Number of patients discontinuing the study because of drug‐related adverse events
In the Gribben 2005 study, one patient (24%) in the alemtuzumab arm discontinued treatment due to adverse events, while in the rituximab arm, there were six patients (75%) (P = 0.22).
The CLL2007FMP trial was stopped prematurely because of an increase in mortality in the alemtuzumab arm (seven patients; 8.5%). In the additional alemtuzumab arm, 59 patients (71.4%) received all six cycles and, in the additional rituximab arm 63 patients (76.5%).
Alemtuzumab versus anti‐leukaemic therapy
One RCT (N = 297), evaluated the efficacy and safety of alemtuzumab compared with chemotherapy (chlorambucil) in patients with relapsed CLL (CAM 307).
Overall survival
CAM 307 did not report a HR or survival curve for OS. Median survival had not yet been reached; 84% of patients were alive in each arm at the data cut‐off or at the last follow‐up date (24.6 months).
Progression‐free survival (PFS)
In CAM 307 (N = 297), alemtuzumab statistically significantly improved PFS compared with chlorambucil (HR 0.58; 95% CI 0.43 to 0.77; P = 0.0001).
Time to next treatment
Patients receiving alemtuzumab had a statistically significant longer median time to alternative treatment (23.3 months) compared with those receiving chlorambucil (14.7 months, P = 0.0001).
Treatment related mortality
CAM 307 reported the following statistical non‐significant information regarding TRM, defined as death occurring on therapy, or within 30 days: one patient (0.6%) died in the alemtuzumab arm versus three patients (2.0%) in the chlorambucil arm (P = 0.34).
Overall response rate (ORR)
Statistically, significantly more patients in the alemtuzumab arm achieved an OR: 124 patients (83.2%) on alemtuzumab versus 82 patients (55.4%) on chlorambucil (P < 0.0001).
Complete response rate (CRR)
There is a statistically significant advantage in terms of CRR for those patients receiving alemtuzumab (36 patients (24.2%) compared with those who received alemtuzumab compared with the three patients (2.0%)) who received chlorambucil (P < 0.0001).
Minimal residual disease (MRD)
The MRD is statistically significantly higher in the alemtuzumab arm (11 patients; 7.4%) versus no patients (0%) in the chlorambucil arm (P = 0.0008).
Adverse events
Detailed adverse events are listed in Table 3. For most adverse events, there is no statistically significant difference in grade 3/4 adverse events between both arms (chills, urticaria, hypotension, rash, nausea, vomiting, anaemia, thrombocytopenia, haemolytic anaemia, febrile neutropenia, and bacteria/sepsis, symptomatic CMV infection). However, there were statistically, significantly more adverse events (all grades) for asymptomatic CMV infection (77 patients (51.7% versus 11 patients (7.4%) and symptomatic CMV infection (23 patients (15.4%) versus no patients (0%)). Patients receiving alemtuzumab statistically significantly developed more grade 3/4 pyrexia (8.2% versus 0%; P = 0.03), neutropenia (41% versus 25%; P = 0.004) and SAEs (39 patients (26.5%) in the alemtuzumab arm compared with 10 patients (6.8%) in the chlorambucil arm (P < 0.0001)).
1. Adverse events (alemtuzumab versus chlorambucil); CAM 307.
| Adverse event | Alemtuzumab arm; N (%) | Chlorambucil arm; N (%) | P value |
| Grade 3/4 pyrexia | 12 (8.2%) | 0 (0%) | 0.03 |
| Grade 3/4 chills | 5 (3.4%) | 0 (0%) | 0.10 |
| Grade 3/4 urticaria | 3 (2.0%) | 0 (0%) | 0.20 |
| Grade 3/4 hypotension | 2 (1.4%) | 0 (0%) | 0.30 |
| Grade 3/4 rash | 1 (0.7%) | 0 (0%) | 0.50 |
| Grade 3/4 asymptomatic CMV with PCR positivity | 6 (4.1%) | 0 (0%) | 0.08 |
| Grade 3/4 symptomatic CMV infection | 6 (4.1%) | 0 (0%) | 0.08 |
| All grades asymptomatic CMV with PCR positivity | 77 (52.4%) | 11 (7.5%) | <0.0001 |
| All grades symptomatic CMV infection | 23 (15.6%) | 0 (0%) | 0.007 |
| Grade 3/4 nausea | 0 (0%) | 1 (0.7%) | 0.50 |
| Grade 3/4 vomiting | 0 (0%) | 1 (0.7%) | 0.50 |
| Grade 3/4 anaemia | 16 (11%) | 26 (18%) | 0.10 |
| Grade 3/4 neutropenia | 60 (41%) | 36 (25%) | 0.004 |
| Grade 3/4 thrombocytopenia | 18 (12%) | 17 (12%) | 0.87 |
| Haemolytic anaemia | 1 (0.7%) | 2 (1.4%) | 0.57 |
| Febrile neutropenia | 7 (4.8%) | 4 (2.7%) | 0.37 |
| Bacteria/sepsis | 4 (3.0%) | 2 (1.4%) | 0.42 |
| SAE | 39 (26.5%) | 10 (6.8%) | <0.0001 |
| Richter's transformation | 0 (0%) | 0 (0%) |
CMV: cytomegalovirus PCR: polymerase chain reaction SAE: serious adverse event
Number of patients discontinuing the study because of drug‐related adverse events
Statistically, significantly more patients in the alemtuzumab arm permanently withdrew from the study because of adverse events (29 patients (19.7%) versus six patients (4.1%) in the chlorambucil arm (P = 0.0003)).
Different dosages or time schedules of alemtuzumab
We did not identify any RCT regarding this comparison.
Discussion
Summary of main results
The following findings emerge from this systematic review, evaluating the role of alemtuzumab in newly diagnosed patients as well as in relapsed patients.
In trials evaluating anti‐leukaemic therapy with alemtuzumab versus identical anti‐leukaemic therapy alone the results are as follows (two trials, N = 356 patients).
OS, PFS and CRR are statistically significantly improved in patients receiving alemtuzumab compared with those not receiving alemtuzumab.
There is no evidence that TRM or ORR is different in patients with CLL receiving additional alemtuzumab compared with those not receiving alemtuzumab.
There is a statistically significantly higher rate of infections in general and CMV reactivation in patients receiving alemtuzumab.
No statistically significant difference is detectable for haematological grade 3 or 4 adverse events and serious adverse events, although one of the two included trials was closed prematurely due to an increase of acute severe infections.
For the comparison of anti‐leukaemic therapy with alemtuzumab versus different anti‐leukaemic therapy without alemtuzumab we found two studies evaluating additional alemtuzumab compared to additional rituximab) (N = 177 patients).
There are no data provided for OS, PFS and time to next treatment.
Due to the small number of patients, no statistically significant difference is seen for TRM, ORR, CRR, and grade 3 or 4 neutropenia.
Statistically, significantly more serious adverse events and serious febrile neutropenia occurred in patients receiving alemtuzumab compared with those receiving rituximab. One of the two included trials had to be stopped early because of an increase in mortality in the alemtuzumab arm.
-
The following results emerge for the comparison of alemtuzumab versus anti‐leukaemic therapy) (one trial; N = 297 patients),
There is no HR reported for OS. Median survival has not yet been reached, 84% of patients were alive in each arm at the data cut‐off or at the last follow‐up date (24.6 months).
Alemtuzumab statistically significantly improves PFS, time to next treatment, ORR, CRR and MRD rate compared with chlorambucil.
No statistically significant differences were visible for most grade 3 or 4 adverse events (chills, urticaria, hypotension, rash, nausea, vomiting, anaemia, thrombocytopenia, haemolytic anaemia, febrile neutropenia, and bacteria/sepsis, symptomatic CMV infection).
Statistically, significantly more adverse events (all grades), more asymptomatic CMV infections and symptomatic CMV infections occurred in the patients treated with alemtuzumab compared with those treated with chlorambucil. Statistically, significantly more patients in the alemtuzumab arm permanently withdrew from the study because of adverse events.
Due to the small number of identified RCTs, subgroup analyses included one trial only in each comparison and are of limited power. We could not detect any difference in the total effect estimate compared with all kinds of subgroups (newly diagnosed versus relapsed patients, first‐line treatment versus consolidation treatment, combined with chemotherapy versus without further chemotherapy).
Overall completeness and applicability of evidence
Five published studies addressed the use of alemtuzumab in patients with CLL and are included in this systematic review and meta‐analysis. However, two trials were published as abstracts only and the full‐text publications are likely to provide more data on relevant outcomes such as, OS, PFS and time to next treatment. It is a serious failure that two trials did not report overall survival (no HR or survival curve), although both trials were published as full‐texts.
The five included trials are clinical heterogenous and we therefore, did not pool them in one meta‐analysis but in three main analyses.
Moreover, we are aware of six ongoing studies. We will include the findings of these trials in an update of this review and they could lead to different conclusions. One trial mentioned as ongoing was terminated prematurely due to a low recruitment rate. Probably no results of this trial will be published, because only one patient was enrolled.
Quality of the evidence
Overall, the quality of the five included trials (845 patients) was moderate. Two included trials were published as abstracts only, therefore, we were not able to assess the potential risk of bias for these trials in detail. All the included trials were reported as randomised and as open‐label studies. One of the included trials reported allocation concealment. The open‐label design and unclear allocation concealment could lead to selection, performance or detection biases. Due to the limited abstract publication of two trials, we judged selective reporting as high for these trials, indicating a potential risk of reporting bias. The premature closure of two trials due to an increased incidence of severe infections or increase in mortality in the alemtuzumab arm could lead to other sources of bias. In one of these trials the strongly improved PFS in patients treated with alemtuzumab could be too good to be true and could be caused by the low number of patients randomised (21 instead of 90 patients). Another potential source of bias is this trial is the uncertainty in the HR calculation. In this trial the HR for PFS was calculated from a survival curve with a constant censoring as described by Tierney 2007.
The robustness of all results was tested by sensitivity and subgroup analysis based on prospectively defined parameters. However, because of the small number of trials included in each analysis, obtaining reliable information from subgroup and sensitivity analyses is unlikely.
Potential biases in the review process
We tried to avoid bias by doing all relevant processes in duplicate. We are not aware of any obvious flaws in our review process.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first comprehensive systematic review and meta‐analysis focusing on the treatment of patients with CLL using the monoclonal antibody alemtuzumab.
Individual non‐randomised studies in patients with CLL reported an improved rate of complete and partial responses compared with historical data that might have an positive effect on overall and progression‐free survival (Byrd 2009a; Hainsworth 2008; Kaufman 2011). However, in accordance with this systematic review, these authors demonstrated an increased rate of CMV reactivation and other opportunistic infections, in some cases even life‐threatening (Byrd 2009a; Hainsworth 2008; Kaufman 2011). Some recent publications have pointed out the requirement of close monitoring for CMV infections and pre‐emptive therapy using intravenous ganciclovir (Byrd 2009a; Elter 2009a).
Faderl et al. showed promising results on CRR for the combination of intravenous alemtuzumab followed by subcutaneous alemtuzumab injection (Faderl 2010). According to some authors, subcutaneous alemtuzumab might be an alternative to intravenous alemtuzumab to treat safely residual disease in patients with CLL without an increased infection rate (Cortelezzi 2009; Stilgenbauer 2009; Wierda 2011).
Authors' conclusions
Implications for practice.
There is currently evidence from one single large trial that alemtuzumab compared to no further therapy improves overall survival in patients with CLL. Progression‐free survival is increased if alemtuzumab is compared with no further therapy; the effect on PFS for the comparison alemtuzumab versus rituximab awaits further evaluation. As examined here, there is insufficient direct evidence from RCTs to recommend alemtuzumab over rituximab. We demonstrated that alemtuzumab statistically significantly increases the rate of CMV reactivations, infections and SAEs.
Implications for research.
More RCTs with the primary endpoint overall survival and a longer follow up period are needed to assess the effect of alemtuzumab on OS. This may be particularly important in situations where PFS is improved to evaluate whether this effect will translate in an OS advantage. Moreover, the optimal dose, schedule and route of alemtuzumab administration should be evaluated in RCTs. To avoid CMV reactivation, CMV prophylaxis or pre‐emptive treatment should be considered in the trial design. Additionally, further research is needed to clarify the efficacy and safety of alemtuzumab compared with other monoclonal antibodies such as rituximab or ofatumumab or, in addition to these antibodies.
Acknowledgements
Verena Roloff was supported by an Medical Research Council studentship.
We are grateful to the following persons for their comments and improving the protocol:
Prof. Lena Specht and Prof. Keith Wheatley, Editors of the Cochrane Haematological Malignancies Group,
Céline Fournier, Consumer Editor of the Cochrane Haematological Malignancies Group,
Sabine Kluge from the Editorial Base of the Cochrane Haematological Malignancies Group for proof reading and Janet Wale for copy editing.
Appendices
Appendix 1. Search strategy CENTRAL
Searches (1990 to March 2009)
MeSH descriptor Leukemia, B‐Cell explode all trees
MeSH descriptor Leukemia, Lymphocytic, Chronic, B‐Cell explode all trees
(leu*em* NEAR/ lymphocyt*) or (leu*em* NEAR/ lymphoblast*) or (leu*em* NEAR/ linfoid*) or (leu*em* NEAR/ b‐cell*)
(leu*em* NEAR/ lymphocyt*) or (leu*em* NEAR/ lymphoblast*) or (leu*em* NEAR/ linfoid*) or (leu*em* NEAR/ b‐cell*)
(lymph* NEAR/ lymphocyt*) or (lymph* NEAR/ lymphoblast*) or (lymph* NEAR/ linfoid*) or (lymph* NEAR/ b‐cell*)
(chronic*) or (cronic*) or (chroniq*) or (well‐differential*)
(#6 AND ( #3 OR #4 OR #5 ))
(lymphom*) and (small cell* or small‐cell*)
(lymphom* NEAR/2 lymphocyt*)
(lymphoplasma*ytoid*)
(cll or sll)
(#1 OR #2 OR #7 OR #8 OR #9 OR #10 OR #11)
Searches (Update March 2009 to 18. April 2011)
MeSH descriptor Leukemia, B‐Cell explode all trees
MeSH descriptor Leukemia, Lymphocytic, Chronic, B‐Cell explode all trees
(leu*em* NEAR/ lymphocyt*) or (leu*em* NEAR/ lymphoblast*) or (leu*em* NEAR/ linfoid*) or (leu*em* NEAR/ b‐cell*)
(leu*em* NEAR/ lymphocyt*) or (leu*em* NEAR/ lymphoblast*) or (leu*em* NEAR/ linfoid*) or (leu*em* NEAR/ b‐cell*)
(lymph* NEAR/ lymphocyt*) or (lymph* NEAR/ lymphoblast*) or (lymph* NEAR/ linfoid*) or (lymph* NEAR/ b‐cell*)
(chronic*) or (cronic*) or (chroniq*) or (well‐differential*)
(#6 AND ( #3 OR #4 OR #5 ))
(lymphom*) and (small cell* or small‐cell*)
(lymphom* NEAR/2 lymphocyt*)
(lymphoplasma*ytoid*)
(cll or sll)
(#1 OR #2 OR #7 OR #8 OR #9 OR #10 OR #11)
MeSH descriptor Antibodies, Monoclonal explode all trees
(antibod* near/2 monoclonal*)
(alemtuzumab*)
(campath*)
(CD52 NEAR/3 antibod*) or (CD‐52 NEAR/3 antibod*) or (CD 52 NEAR/3 antibod*)
(ANTI‐CD52 or ANTI CD52)
(#13 OR #14 OR #15 OR #16 OR #17 OR #18)
(#12 AND #19)
Appendix 2. Search strategy (Ovid MEDLINE)
Search strategy (1990 to March 2009)
exp Leukemia, B‐Cell/
exp Leukemia, Lymphocytic, Chronic, B‐Cell/
((leuk?em$ or leu?em$ or lymph$) adj (lymphocyt$ or lymphoblast$ or linfoid$ or b‐cell$)).tw,kf,ot.
(chronic$ or cronic$ or chroniq$ or well‐differential$).tw,kf,ot. (589526)
3 and 4
(lymphom$ and (small cell$ or small‐cell$)).tw,kf,ot.
(lymphom$ adj2 lymphocyt$).tw,kf,ot.
lymphoplasma?ytoid.tw,kf,ot.
cll.tw.
sll.tw.
or/6‐10
1 or 2 or 5 or 11
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/13‐20
humans.sh.
21 and 22
12 and 23
Search strategy (Update March 2009 to April 2010)
exp B CELL LEUKEMIA/
exp CHRONIC LYMPHATIC LEUKEMIA/
((leuk?em$ or leu?em$ or lymph$) adj (lymphocyt$ or lymphoblast$ or linfoid$ or b‐cell$)).tw,kf,ot.
(chronic$ or cronic$ or chroniq$ or well‐differential$).tw,kf,ot.
3 and 4
(lymphom$ and (small cell$ or small‐cell$)).tw,kf,ot.
(lymphom$ adj2 lymphocyt$).tw,kf,ot.
lymphoplasma?ytoid.tw,kf,ot.
cll.tw.
sll.tw.
or/6‐10
1 or 2 or 5 or 11
exp ANTIBODIES, MONOCLONAL/
(antibod$ adj2 monoclonal$).tw,kf,ot.
13 or 14
alemtuzumab$.tw,kf,ot,nm.
campath$.tw,kf,ot.
((CD52 or CD‐52 or CD 52) adj3 antibod$).tw,kf,ot,nm.
(ANTI‐CD52 or ANTI CD52).tw,kf,ot,nm.
or/16‐19
12 and 20
12 and (15 or 20)
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/23‐30
humans.sh.
31 and 32
22 and 33
Search strategy (Update April 2010 to 18. November 2011)
exp LEUKEMIA, B‐CELL/
exp LEUKEMIA, LYMPHOCYTIC, CHRONIC, B‐CELL/
((leuk?em$ or leu?em$ or lymph$) adj (lymphocyt$ or lymphoblast$ or linfoid$ or b‐cell$)).tw,kf,ot.
(chronic$ or cronic$ or chroniq$ or well‐differential$).tw,kf,ot.
3 and 4
(lymphom$ and (small cell$ or small‐cell$)).tw,kf,ot.
(lymphom$ adj2 lymphocyt$).tw,kf,ot.
lymphoplasma?ytoid.tw,kf,ot.
cll.tw.
sll.tw.
or/6‐10
1 or 2 or 5 or 11
exp ANTIBODIES, MONOCLONAL/
(antibod$ adj2 monoclonal$).tw,kf,ot.
13 or 14
alemtuzumab$.tw,kf,ot,nm.
campath$.tw,kf,ot.
((CD52 or CD‐52 or CD 52) adj3 antibod$).tw,kf,ot,nm.
(ANTI‐CD52 or ANTI CD52).tw,kf,ot,nm.
or/16‐19
12 and 20
12 and (15 or 20)
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/23‐30
humans.sh.
31 and 32
21 and 33
22 and 33
limit 35 to ed=20100401‐20111118
Appendix 3. Search strategy (EMBASE)
Searches (1990 to March 2009)
exp B CELL LEUKEMIA/
exp CHRONIC LYMPHATIC LEUKEMIA/
((leuk?em$ or leu?em$ or lymph$) adj (lymphocyt$ or lymphoblast$ or linfoid$ or b‐cell$)).tw.
(chronic$ or cronic$ or chroniq$ or well‐differential$).tw.
3 and 4
(lymphom$ and (small cell$ or small‐cell$)).tw.
(lymphom$ adj2 lymphocyt$).tw.
lymphoplasma?ytoid.tw.
cll.tw.
sll.tw.
or/6‐10
1 or 2 or 5 or 11
(random$ or placebo$).ti,ab.
((single$ or double$ or triple$ or treble$) and (blind$ or mask$)).ti,ab.
controlled clinical trial$.ti,ab.
RETRACTED ARTICLE/
or/13‐16
(animal$ not human$).sh,hw.
17 not 18
12 and 19
Searches (Update March 2009 to 2010)
exp LEUKEMIA, B‐CELL/
exp LEUKEMIA, LYMPHOCYTIC, CHRONIC, B‐CELL/
((leuk?em$ or leu?em$ or lymph$) adj (lymphocyt$ or lymphoblast$ or linfoid$ or b‐cell$)).tw.
(chronic$ or cronic$ or chroniq$ or well‐differential$).tw.
3 and 4
(lymphom$ and (small cell$ or small‐cell$)).tw.
(lymphom$ adj2 lymphocyt$).tw.
lymphoplasma?ytoid.tw.
cll.tw.
sll.tw.
or/6‐10
1 or 2 or 5 or 11
exp MONOCLONAL ANTIBODY/
(antibod$ adj2 monoclonal$).tw.
ALEMTUZUMAB/
alemtuzumab$.tw.
campath$.tw.
((CD52 or CD‐52 or CD 52) adj3 antibod$).tw.
(ANTI‐CD52 or ANTI CD52).tw.
or/15‐19
12 and 20
12 and (13 or 14 or 20)
(random$ or placebo$).ti,ab.
((single$ or double$ or triple$ or treble$) and (blind$ or mask$)).ti,ab.
controlled clinical trial$.ti,ab.
RETRACTED ARTICLE/
or/23‐26
(animal$ not human$).sh,hw.
27 not 28
22 and 29
Data and analyses
Comparison 1. Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PFS ‐ overall analysis | 2 | 356 | Hazard Ratio (Fixed, 95% CI) | 0.58 [0.44, 0.76] |
| 2 PFS ‐ subgrouped by treatment regimens | 2 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 combinations with chemotherapy | 1 | 335 | Hazard Ratio (Fixed, 95% CI) | 0.61 [0.47, 0.81] |
| 2.2 not combined with another chemotherapy | 1 | 21 | Hazard Ratio (Fixed, 95% CI) | 0.17 [0.05, 0.60] |
| 3 PFS ‐ subgrouped by starting point of alemtuzumab | 2 | 356 | Hazard Ratio (Fixed, 95% CI) | 0.58 [0.44, 0.76] |
| 3.1 relapse therapy | 1 | 335 | Hazard Ratio (Fixed, 95% CI) | 0.61 [0.47, 0.81] |
| 3.2 consolidation therapy | 1 | 21 | Hazard Ratio (Fixed, 95% CI) | 0.17 [0.05, 0.60] |
| 4 Treatment related mortality | 2 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.17, 1.90] |
| 5 ORR ‐ overall analysis | 2 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.99, 1.23] |
| 6 ORR ‐ subgrouped by treatment regimens | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 combinations with chemotherapy | 1 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.97, 1.21] |
| 6.2 not combined with another chemotherapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.92, 2.14] |
| 7 ORR ‐ subgrouped by starting point of alemtuzumab | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 relapse therapy | 1 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.97, 1.21] |
| 7.2 consolidation therapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.92, 2.14] |
| 8 CRR ‐ overall analysis | 2 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.61 [1.26, 5.42] |
| 9 CRR ‐ subgrouped by treatment regimens | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 combinations with chemotherapy | 1 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [1.30, 6.83] |
| 9.2 not combined with another chemotherapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.28, 6.56] |
| 10 CRR ‐ subgrouped by starting point of alemtuzumab | 2 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.61 [1.26, 5.42] |
| 10.1 relapse therapy | 1 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [1.30, 6.83] |
| 10.2 consolidation therapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.28, 6.56] |
| 11 CMV reactivation ‐ overall analysis | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.52 [1.42, 77.68] |
| 12 CMV reactivation ‐ subgrouped by treatment regimens | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 combinations with chemotherapy | 1 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.05 [0.49, 166.84] |
| 12.2 not combined with another chemotherapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.92 [0.76, 187.84] |
| 13 CMV reactivation ‐ subgrouped by starting point of alemtuzumab | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.52 [1.42, 77.68] |
| 13.1 relapse therapy | 1 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.05 [0.49, 166.84] |
| 13.2 consolidation therapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.92 [0.76, 187.84] |
| 14 Infections (all grades) ‐ overall analysis | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.01, 1.74] |
| 15 Infections (all grades) ‐ subgrouped by treatment regimens | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 combinations with chemotherapy | 1 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.88, 1.53] |
| 15.2 not combined with another chemotherapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.25 [1.27, 291.20] |
| 16 Infections (all grades) ‐ subgrouped by starting point of alemtuzumab | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 relapse therapy | 1 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.88, 1.53] |
| 16.2 consolidation therapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.25 [1.27, 291.20] |
| 17 Anaemia grade 3/4 ‐ overall analysis | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.33, 1.20] |
| 18 Anaemia grade 3/4 ‐ subgrouped by treatment regimens | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 combinations with chemotherapy | 1 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.26, 1.06] |
| 18.2 not combined with another chemotherapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.58 [0.25, 85.33] |
| 19 Anaemia grade 3/4 ‐ subgrouped by starting point of alemtuzumab | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 relapse therapy | 1 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.26, 1.06] |
| 19.2 consolidation therapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.58 [0.25, 85.33] |
| 20 Neutropenia grade 3/4 ‐ overall analysis | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.97, 1.61] |
| 21 Neutropenia grade 3/4 ‐ subgrouped by treatment regimens | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 combinations with chemotherapy | 1 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.89, 1.48] |
| 21.2 not combined with another chemotherapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.75 [0.88, 213.65] |
| 22 Neutropenia grade 3/4 ‐ subgrouped by starting point of alemtuzumab | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 relapse therapy | 1 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.89, 1.48] |
| 22.2 consolidation therapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.75 [0.88, 213.65] |
| 23 Thrombocytopenia grade 3/4 ‐ overall analysis | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.58, 1.89] |
| 24 Thrombocytopenia grade 3/4 ‐ subgrouped by treatment regimens | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 combinations with chemotherapy | 1 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.45, 1.59] |
| 24.2 not combined with another chemotherapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.25 [0.50, 136.33] |
| 25 Thrombocytopenia grade 3/4 ‐ subgrouped by starting point of alemtuzumab | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 relapse therapy | 1 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.45, 1.59] |
| 25.2 consolidation therapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.25 [0.50, 136.33] |
| 26 SAEs ‐ overall analysis | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.95, 1.89] |
| 27 SAEs ‐ subgrouped by treatment regimens | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 combinations with chemotherapy | 1 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.94, 1.87] |
| 27.2 not combined with another chemotherapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.12, 60.70] |
| 28 SAEs ‐ subgrouped by starting point of alemtuzumab | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 relapse therapy | 1 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.94, 1.87] |
| 28.2 consolidation therapy | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.12, 60.70] |
1.1. Analysis.
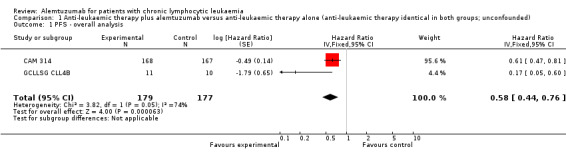
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 1 PFS ‐ overall analysis.
1.2. Analysis.
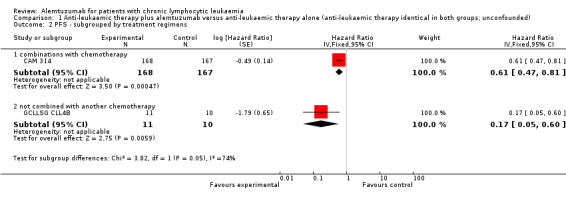
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 2 PFS ‐ subgrouped by treatment regimens.
1.3. Analysis.
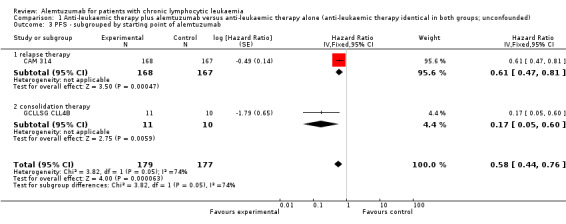
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 3 PFS ‐ subgrouped by starting point of alemtuzumab.
1.4. Analysis.
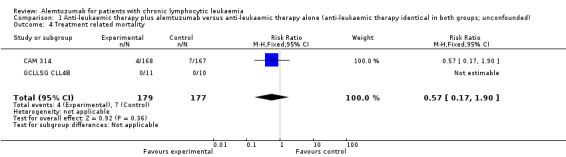
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 4 Treatment related mortality.
1.5. Analysis.
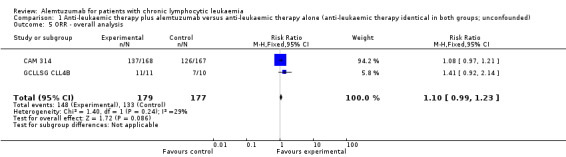
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 5 ORR ‐ overall analysis.
1.6. Analysis.
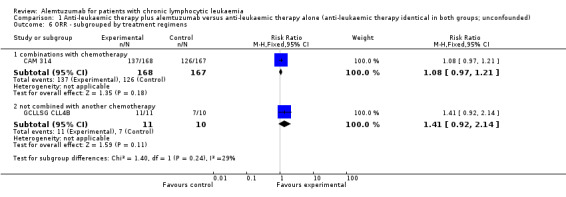
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 6 ORR ‐ subgrouped by treatment regimens.
1.7. Analysis.
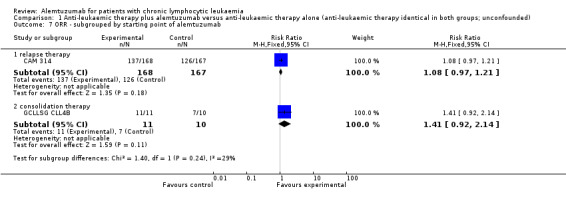
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 7 ORR ‐ subgrouped by starting point of alemtuzumab.
1.8. Analysis.
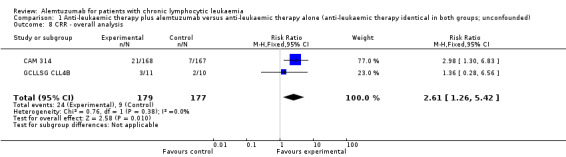
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 8 CRR ‐ overall analysis.
1.9. Analysis.
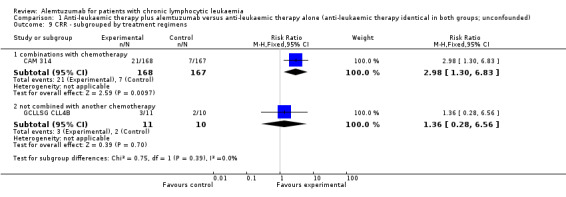
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 9 CRR ‐ subgrouped by treatment regimens.
1.10. Analysis.
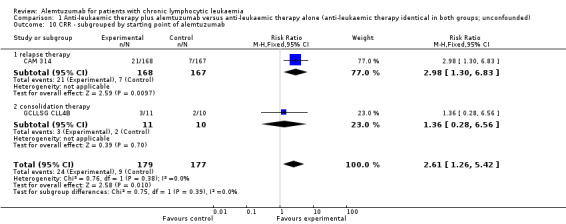
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 10 CRR ‐ subgrouped by starting point of alemtuzumab.
1.11. Analysis.
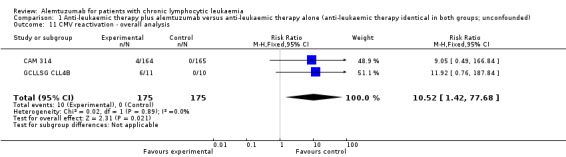
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 11 CMV reactivation ‐ overall analysis.
1.12. Analysis.
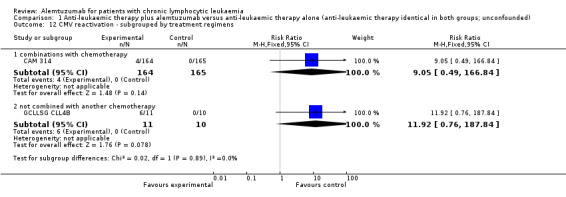
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 12 CMV reactivation ‐ subgrouped by treatment regimens.
1.13. Analysis.
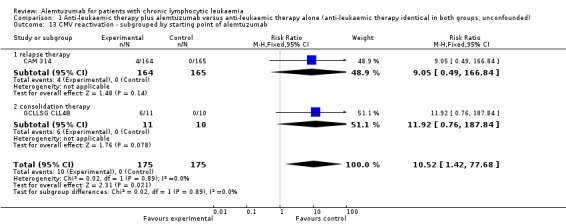
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 13 CMV reactivation ‐ subgrouped by starting point of alemtuzumab.
1.14. Analysis.
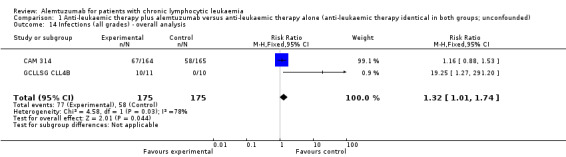
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 14 Infections (all grades) ‐ overall analysis.
1.15. Analysis.
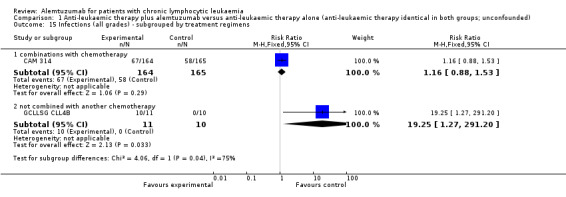
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 15 Infections (all grades) ‐ subgrouped by treatment regimens.
1.16. Analysis.
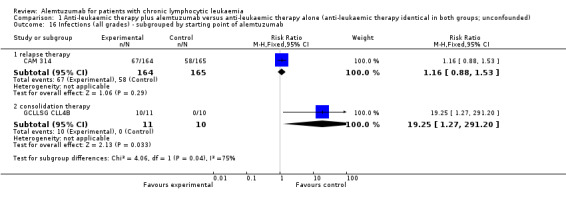
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 16 Infections (all grades) ‐ subgrouped by starting point of alemtuzumab.
1.17. Analysis.
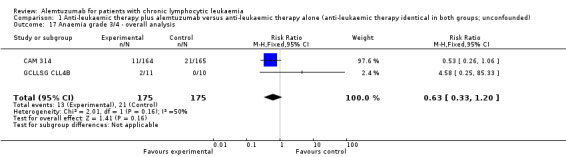
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 17 Anaemia grade 3/4 ‐ overall analysis.
1.18. Analysis.
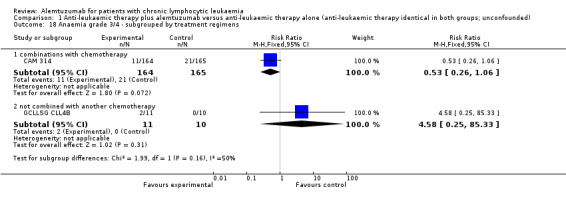
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 18 Anaemia grade 3/4 ‐ subgrouped by treatment regimens.
1.19. Analysis.
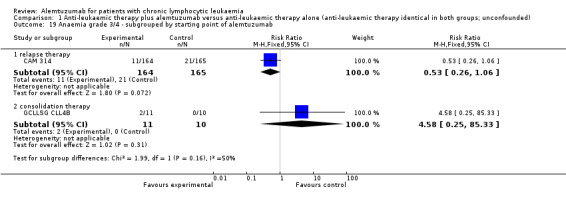
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 19 Anaemia grade 3/4 ‐ subgrouped by starting point of alemtuzumab.
1.20. Analysis.
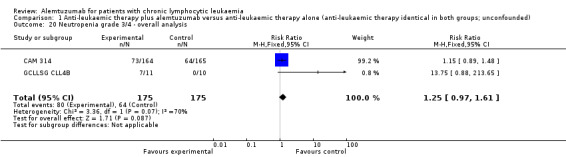
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 20 Neutropenia grade 3/4 ‐ overall analysis.
1.21. Analysis.
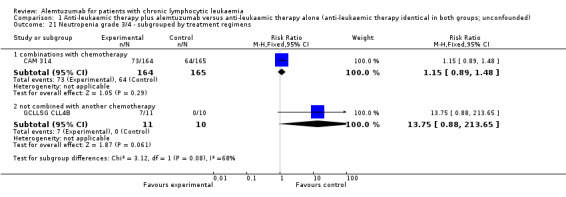
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 21 Neutropenia grade 3/4 ‐ subgrouped by treatment regimens.
1.22. Analysis.
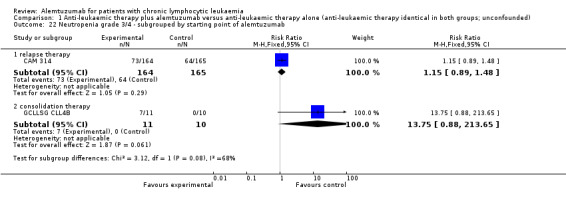
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 22 Neutropenia grade 3/4 ‐ subgrouped by starting point of alemtuzumab.
1.23. Analysis.
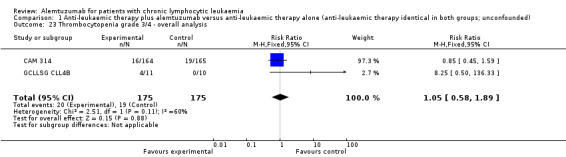
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 23 Thrombocytopenia grade 3/4 ‐ overall analysis.
1.24. Analysis.
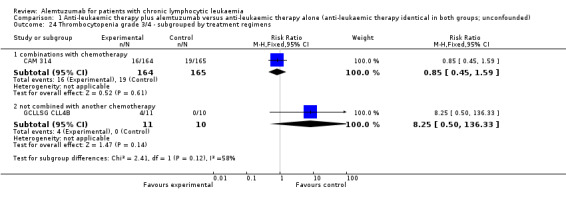
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 24 Thrombocytopenia grade 3/4 ‐ subgrouped by treatment regimens.
1.25. Analysis.
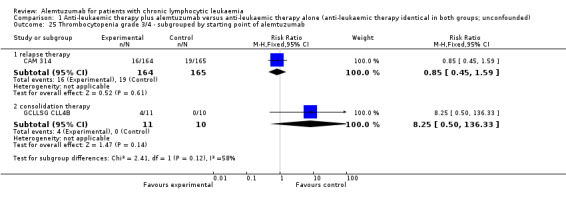
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 25 Thrombocytopenia grade 3/4 ‐ subgrouped by starting point of alemtuzumab.
1.26. Analysis.
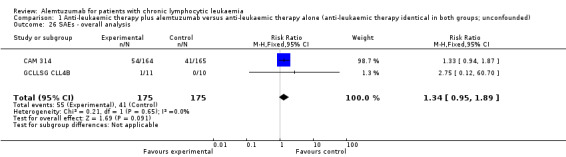
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 26 SAEs ‐ overall analysis.
1.27. Analysis.
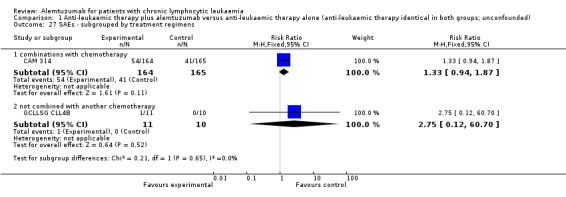
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 27 SAEs ‐ subgrouped by treatment regimens.
1.28. Analysis.
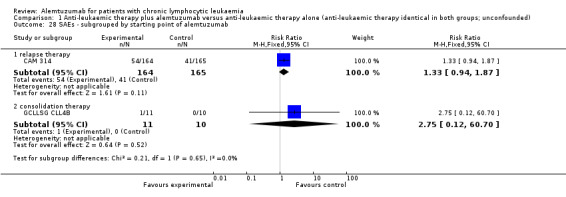
Comparison 1 Anti‐leukaemic therapy plus alemtuzumab versus anti‐leukaemic therapy alone (anti‐leukaemic therapy identical in both groups; unconfounded), Outcome 28 SAEs ‐ subgrouped by starting point of alemtuzumab.
Comparison 2. Anti‐leukaemic therapy with alemtuzumab versus anti‐leukaemic therapy without alemtuzumab (anti‐leukaemic therapy not identical in both groups, confounded).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treament related mortality | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.20 [0.66, 15.50] |
| 2 Treatment related mortality ‐ subgrouped by alemtuzumab treatment regiment | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.20 [0.66, 15.50] |
| 2.1 first‐line therapy | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.11 [0.50, 166.53] |
| 2.2 relapse therapy | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 8.00] |
| 3 ORR ‐ overall analysis | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.07] |
| 4 ORR ‐ subgrouped by alemtuzumab treatment regimen | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 first‐line therapy | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.04] |
| 4.2 relapse therapy | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.63, 4.88] |
| 5 CRR ‐ overall analysis | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.67, 1.08] |
| 6 CRR ‐ subgrouped by alemtuzumab treatment regimens | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.67, 1.08] |
| 6.1 first‐line therapy | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.03] |
| 6.2 relapse therapy | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.5 [0.45, 27.52] |
2.1. Analysis.
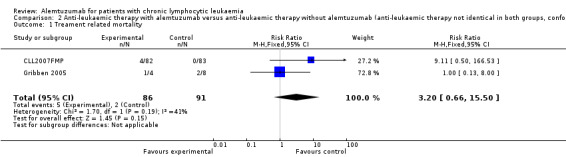
Comparison 2 Anti‐leukaemic therapy with alemtuzumab versus anti‐leukaemic therapy without alemtuzumab (anti‐leukaemic therapy not identical in both groups, confounded), Outcome 1 Treament related mortality.
2.2. Analysis.
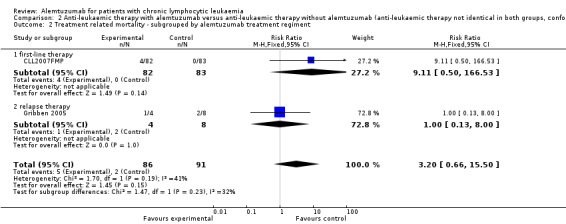
Comparison 2 Anti‐leukaemic therapy with alemtuzumab versus anti‐leukaemic therapy without alemtuzumab (anti‐leukaemic therapy not identical in both groups, confounded), Outcome 2 Treatment related mortality ‐ subgrouped by alemtuzumab treatment regiment.
2.3. Analysis.
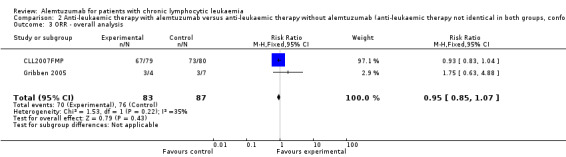
Comparison 2 Anti‐leukaemic therapy with alemtuzumab versus anti‐leukaemic therapy without alemtuzumab (anti‐leukaemic therapy not identical in both groups, confounded), Outcome 3 ORR ‐ overall analysis.
2.4. Analysis.
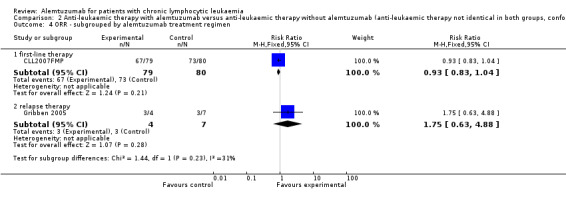
Comparison 2 Anti‐leukaemic therapy with alemtuzumab versus anti‐leukaemic therapy without alemtuzumab (anti‐leukaemic therapy not identical in both groups, confounded), Outcome 4 ORR ‐ subgrouped by alemtuzumab treatment regimen.
2.5. Analysis.
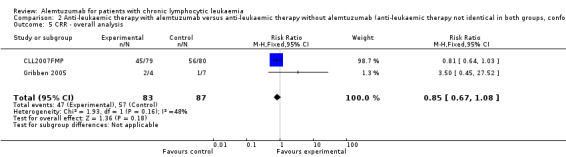
Comparison 2 Anti‐leukaemic therapy with alemtuzumab versus anti‐leukaemic therapy without alemtuzumab (anti‐leukaemic therapy not identical in both groups, confounded), Outcome 5 CRR ‐ overall analysis.
2.6. Analysis.
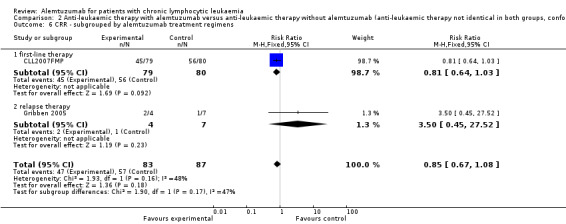
Comparison 2 Anti‐leukaemic therapy with alemtuzumab versus anti‐leukaemic therapy without alemtuzumab (anti‐leukaemic therapy not identical in both groups, confounded), Outcome 6 CRR ‐ subgrouped by alemtuzumab treatment regimens.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CAM 307.
| Methods | Randomisation
Recruitment period
Median follow‐up time
|
|
| Participants | Eligibility criteria
Patients (N = 297)
Mean age
Gender (male)
Stage of disease (Rai)
Country
|
|
| Interventions | Alemtuzumab
Chlorambucil (every 28 days)
Treatment was to be discontinued in both arms if a patient experienced progressive disease, unacceptable toxicity, autoimmune anaemia, or autoimmune thrombocytopenia. Subsequent treatment was at the discretion of the treating physician. |
|
| Outcomes | Reported
Primary outcome:
Secondary outcomes
|
|
| Notes | All authors are either employees or consultants for Genzyme Corp or have received research grants from Genzyme Corp. One author is a consultant or has a advisory role for Bayer Schering Pharma. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned" Comment: The authors did not describe the method used to generate the allocation sequence. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) overall survival | Unclear risk | Comment: The study did not report this outcome. |
| Blinding (performance bias and detection bias) All other outcomes | Unclear risk | Comment: Patient and physician unblinded. Quote (for outcome assessor): "All the efficacy analyses were based on the independent response review panel's assessment of eligibility, response and, data of progression". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 297 recruited patients Quote: "Three patients (two assigned to alemtuzumab and one to chlorambucil) withdrew consent before treatment administration and were not included in the safety analysis." Quote: "All randomly assigned patients were included in the efficacy analysis per the intent‐to‐treat principle." |
| Selective reporting (reporting bias) | Unclear risk | Comment: A study protocol is registered (clinical.trials.gov: NCT00046683), but it does not provide any information of the pre‐planned primary and secondary outcomes. |
| Other bias | Unclear risk | Comment: The study appears to be free of other sources of bias. |
CAM 314.
| Methods | Randomisation
Recruitment period
Median follow‐up time
|
|
| Participants | Eligibility criteria
Patients randomised (N = 335)
Mean age
Gender (male):
Stage of disease (Rai stage group)
Country
|
|
| Interventions | Flu‐Cam (every 28 days; up to 6 cycles)
Flu (every 28 days, up to 6 cycles)
All patients received prophylaxis with cotrimoxazole and famciclovir until CD4+ counts were > 200 cells/mcL. |
|
| Outcomes | Primary outcome
Secondary outcomes
Reported (relevant for this review)
Not reported (relevant for this review)
Not evaluated (relevant for this review)
|
|
| Notes | Most of the authors are either employees or consultants for Genzyme Corp or have received research grants from Genzyme Corp. Two authors are employees of Genzyme Corp. More patients than planned were randomised without a clear rationale. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients (..) were randomized to FluCam or Flu using the minimization method" Comment: The authors used a adequate method to generate the allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: "Computer‐generated allocation schedule" |
| Blinding (performance bias and detection bias) overall survival | Low risk | Comment: The review authors judge that the outcome OS in this unblinded trial is unlikely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) All other outcomes | Unclear risk | Comment: Patient and physician unblinded. No information about blinding of outcome assessor provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Analysis was by intention to treat" Comment: all randomised patients were analysed |
| Selective reporting (reporting bias) | Low risk | Preplanned outcomes (ClinicalTrials.gov: NCT00086580) in the publication for all pre‐defined outcomes reported except
Comment: The primary endpoint and the patient‐important outcomes except quality of life are reported |
| Other bias | Unclear risk | Quote: "The planned sample size for this study of 300 patients..." "... 335 patients were enrolled..." "More patients than planned were enrolled to enable an analysis of potential drug‐drug interactions." Comment: The rationale for randomising more than the planned number of patients is unclear |
CLL2007FMP.
| Methods | Randomisation
Recruitment period
Median follow‐up time
|
|
| Participants | Eligibility criteria
Patients randomised (N = 165)
The trial was stopped early due to unacceptable toxicity in the FluC‐Cam arm (6 deaths versus 0 in FluC‐R arm) Mean age
Gender (male, female)
Stage of disease (Rai stage group)
Countries
|
|
| Interventions | FluC‐Cam (every 28 days; up to 6 cycles)
FluC‐R
Anti‐infective prophylaxis included trimethoprim‐sulfamethoxazole and valaciclovir during immunochemotherapy and until the CD4‐positive lymphocyte count reached 0.2 109/L. |
|
| Outcomes | Primary outcome
Secondary outcomes
Reported (relevant for this review)
Not reported (relevant for this review)
Not evaluated (relevant for this review)
|
|
| Notes | The trial was discontinued after randomisation of 165 patients for unacceptable toxicity in the FluC‐Cam arm (6 deaths versus 0 in FluC‐R arm). The last 13 patients enrolled were not randomised. The authors stated that they had no relevant conflict of interest to declare |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized to" Comment: The authors did not describe the method used to generate the allocation sequence. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) overall survival | Unclear risk | Comment: The study did not report this outcome. |
| Blinding (performance bias and detection bias) All other outcomes | Unclear risk | Comment: Patient and physician unblinded. No information about blinding of outcome assessor provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote:"165 patients were randomized to (...) R (N = 83 (...)) or Cam (N = 82)"; "Clinical responses were as follows: CR (FCR: 56/80 = 70%, FCCam: 45/79 = 59%, ns)" Reasons of exclusions are not provided. |
| Selective reporting (reporting bias) | High risk | Pre‐planned outcomes (ClinicalTrials.gov: NCT00564512) in the abstract‐publications reported for all pre‐defined outcomes except
|
| Other bias | High risk | Quote: "The trial recruitment was discontinued because of an increase in mortality in the FCCam arm (6 deaths versus 0 in FCR arm), and the last 13 patients enrolled were not randomized" Comment: The trial was stopped early due to data‐dependent process. |
GCLLSG CLL4B.
| Methods | Randomisation
Recruitment period
Median follow‐up time
|
|
| Participants | Eligibility criteria
Patients recruited (N = 23) Two patients refused initiation of study treatment after randomisation and were excluded from analysis
Mean age
Gender (male)
Stage of disease (according to Rai)
Countries
|
|
| Interventions | Patients received six cycles of fludarabine (25 mg/m2 days 1 to 5 IV every 28 days) or fludarabine/cyclophosphamide (fludarabine 30 mg/m2 d1 to 3 IV, cyclophosphamide 250 mg/m2 days 1 to 3 IV every 28 days). Patients were stratified according to induction treatment and response to induction treatment and randomised for treatment with alemtuzumab or observation. Alemtuzumab
Therapy was discontinued, if an unacceptable toxicity occurred and stopping criteria for the trial were set as grade 3 or 4 infections occurring in the alemtuzumab arm in five of the first 10 patients. Premedication with antihistamines (e.g., 2 mg IV clemastin), paracetamol (500 mg PO) and prednisone (100 mg IV) was given with the first dose at each escalation and thereafter only if clinically indicated. Anti‐infective prophylaxis including cotrimoxazole (960 mg PO twice daily, three times per week) and famciclovir (250 mg PO, twice daily) was given during and up to a minimum of 2 months following the discontinuation of alemtuzumab therapy. Control
|
|
| Outcomes | Reported Primary outcome
Secondary outcomes
Not reported
|
|
| Notes | The study was supported by a research grant of Schering AG, Berlin and MedacSchering Onkologie, Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized to" Comment: The authors did not describe the method used to generate the allocation sequence. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) overall survival | Unclear risk | Comment: The study did not report this outcome. |
| Blinding (performance bias and detection bias) All other outcomes | Unclear risk | Comment: Patient and physician unblinded. No information about blinding of outcome assessor provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "In all, 23 patients were recruited for this study, two patients refused initiation of study treatment after randomisation and were excluded from analysis." Comment: It is unclear in which group the two patients (8.7%) were randomised, why they refused treatment according to randomisation and why they were not analysed as randomised. |
| Selective reporting (reporting bias) | Unclear risk | Comment: The study has no registered study protocol. The review authors have not information to permit judgement. |
| Other bias | High risk | Quote: "This randomized phase III trial shows that a consolidation with alemtuzumab in a standard dose of 30mg IV TIW in CLL patients responding to fludarabine‐based chemotherapy is associated with an increased incidence of severe infections and myelotoxicity. Therefore, this multicenter trial was prematurely closed." |
Gribben 2005.
| Methods | Randomisation
Recruitment period
Median follow‐up time
|
|
| Participants | Eligibility criteria
Patients recruited (N = 12)
Mean age
Gender
Stage of disease (Rai stage group)
Country
|
|
| Interventions | Patients were assessed monthly for response while on therapy, and interim restaging occurred at cycle 4. Those who achieved a CR received no further therapy, whereas those who achieved a PR or SD received 2 additional cycles. Flu‐Cam
Flu‐R
|
|
| Outcomes | Outcomes Reported
Not evaluated or reported
|
|
| Notes | No conflict of interest statement in the abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized to" Comment: The authors did not describe the method used to generate the allocation sequence. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) overall survival | Unclear risk | Comment: The study did not report this outcome. |
| Blinding (performance bias and detection bias) All other outcomes | Unclear risk | Comment: Patient and physician unblinded. No information about blinding of outcome assessor provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The information about completeness of outcome data is insufficient to permit judgement. |
| Selective reporting (reporting bias) | High risk | Preplanned outcomes (ClinicalTrials.gov: NCT00086775) in the abstract‐publications reported for all pre‐defined outcomes except:
|
| Other bias | Unclear risk | In this phase II trial 12 patients only were randomised, without providing a rationale for the uneven distribution (4 patients alemtuzumab arm versus 8 patients in the rituximab arm). Comment: The review authors have no further information on the uneven distribution to permit judgement. |
CLL: Chronic lymphocytic leukaemia; CMV: cytomegalovirus; CNS: central nervous system; CR: complete response; CRR: complete response rate; HIV: human immunodeficiency virus; IV: intravenous; MRD: minimal residual disease; ORR: overall response rate; OS: overall survival; PCR: polymerase chain reaction; PFS: progression‐free survival; PO: orally; PR: partial response; TRM: treatment‐related mortality; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bolli 2004 | Not a randomised controlled trial. |
| Byrd 2009 | Not a randomised controlled trial. |
| Elter 2009 | Not a randomised controlled trial. |
| Faderl 2010 | Not a randomised controlled trial. |
| Hale 2004 | Not a randomised controlled trial. |
| Karlsson 2006 | Not a randomised controlled trial. |
| Kennedy 2000 | Not a randomised controlled trial. |
| Lin 2010 | Not a randomised controlled trial. |
| Osterborg 1996 | Not a randomised controlled trial. |
| Pettitt 2006 | Not a randomised controlled trial. |
Characteristics of ongoing studies [ordered by study ID]
2006‐0767.
| Trial name or title | Alemtuzumab + rituximab consolidation in CLL (NCT00771602) |
| Methods | Consolidation therapy for patients with CLL with evidence of residual disease following prior chemo(immuno)therapy. Randomisation:
|
| Participants | Inclusion Criteria
|
| Interventions | Rituximab
Alemtuzumab
Rituximab plus alemtuzumab
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | August 2008 |
| Contact information | The University of Texas M.D. Anderson Cancer Center (Stefan Faderl M.D./ Associate Professor ) |
| Notes | Sponsor: Genzyme Estimated enrolment: 100 Estimated primary completion date: December 2010 (Final data collection date for primary outcome measure) Study status according to ClinicalTrials.gov: This study is terminated ‐ 1 patient enrolled |
39338.
| Trial name or title | Low dose alemtuzumab for consolidation and maintenance of patients with B‐cell CLL (NCT00336206) |
| Methods | SC injection of low dose alemtuzumab for consolidation and maintenance of patients in clinical response after having achieved partial or complete remission after 1st or 2nd line anti‐tumour therapy for B‐Cell CLL Randomisation
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Alemtuzumab
Placebo |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | June 12, 2006 |
| Contact information | Contact: Jorgen Kristensen, MD PhD jkr@emirates.net.ae |
| Notes | Estimated enrolment: 60 Estimated primary completion date: October 2009 Study status according to ClinicalTrials.gov: The recruitment status is unknown because the information has not been verified recently |
CAM203.
| Trial name or title | Subcutaneous alemtuzumab (CAMPATH®, MabCampath®) in relapsed/refractory B‐CLL |
| Methods | A phase II trial to evaluate the efficacy and safety of SC administered alemtuzumab in patients with previously treated B‐CLL. Randomisation
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Alemtuzumab (dose escalation)
Alemtuzumab (no escalation)
|
| Outcomes | Primary outcome
Secondary outcome
|
| Starting date | May 18, 2006 |
| Contact information | Genzyme |
| Notes | Sponsor: Genzyme Estimated enrolment: 85 Estimated primary completion date: July 2011 (Final data collection date for primary outcome measure) Study status according to ClinicalTrials.gov: This study is ongoing, but not recruiting participants. |
CLL8.
| Trial name or title | CLL8: a randomised, phase III study to assess alemtuzumab consolidation therapy in patients with CLL who have responded to previous therapy (ISRCTN63375144) |
| Methods | The trial is intended to assess the effect on PFS of SC alemtuzumab in B‐CLL patients who have responded to previous chemotherapy. Randomisation
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Alemtuzumab
No consolidation therapy
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | June 2008 |
| Contact information | Prof Peter Hillmen Department of Haematology Level 3 Bexley Wing St James's University Hospital Beckett Street Leeds United Kingdom: peter.hillmen@nhs.net |
| Notes | Sponsor: Leeds Teaching Hospitals NHS Trust (UK) Target number of participants: 288 Anticipated end date: December 214 |
DMS‐F0334.
| Trial name or title | Fludarabine combined with either alemtuzumab or rituximab in treating patients with refractory or relapsed B‐CLL (NCT00086775) |
| Methods | Phase II trial comparing combination treatment with fludarabine and alemtuzumab to fludarabine and rituximab in patients with B‐CLL requiring treatment after first line therapy Randomisation
|
| Participants | Inclusion criteria
|
| Interventions | Fludarabine plus alemtuzumab
Fludarabine plus rituximab
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | July 2003 |
| Contact information | Ann S. LaCasce, MD, Dana‐Farber Cancer Institute |
| Notes | Sponsor: Bayer Estimated enrolment: no information provided Estimated primary completion date: June 2005 (Final data collection date for primary outcome measure) Study status according to ClinicalTrials.gov: This study has been completed |
Hovon 68 CLL.
| Trial name or title | A randomised phase III study in previously untreated patients with biological high‐risk CLL: fludarabine and cyclophosphamide (FC) versus FC and low‐dose alemtuzumab (ISRCTN25180151) |
| Methods | Prospective, multicenter, randomised controlled trial Randomisation
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Fludarabine and cyclophosphamide plus alemtuzumab
Fludarabine and cyclophosphamide
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | December 2005 |
| Contact information | Prof M.H.J. Oers, van Academic Medical Center Department of Hematologie P.O. Box 22660 Amsterdam Netherlands m.h.vanoers@amc.uva.nl |
| Notes | Sponsor: Rigshospitalet (Denmark) Target number of participants: 300 Status of trial: completed |
P04715.
| Trial name or title | SCH 727965 in patients with mantle cell lymphoma or B‐CLL (study P04715AM2) (NCT00871546) |
| Methods | A randomised phase 2 study of SCH 727965 in subjects with relapsed or refractory mantle cell lymphoma (MCL) or B‐cell CLL Randomisation
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | B‐CLL patients
MCL patients
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | March 2009 |
| Contact information | No contacts provided |
| Notes | Sponsor: Schering‐Plough Estimated enrolment: 200 Estimated primary completion date: March 2011 (Final data collection date for primary outcome measure) Study status according to ClinicalTrials.gov: This study is ongoing, but not recruiting participants. |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; B‐CLL: B‐cell chronic lymphocytic leukaemia: CLL: chronic lymphocytic leukaemia; CR: complete response; CRR: complete response rate; IV: intravenous; MRD: minimal residual disease; nCR: near complete response; ORR: overall response rate; OS: overall survival; PFS: progression‐free survival; PLL: prolymphocytic leukaemia; PR: partial response; SC: subcutaneous; SCT: stem cell transplantation; SLL: small lymphocytic lymphoma.
Differences between protocol and review
None
Contributions of authors
Nicole Skoetz (NS): methodological expertise, wrote the first and final draft of the review
Kathrin Bauer (KB): data selection, data extraction, proof read and commented on the first and final draft
Ina Monsef (IM): developed the search strategies and ran the database searches, provided reference databases
Thomas Elter (TE): provided clinical expertise, proof read and commented on final draft
Verena Roloff (VR): statistical expertise, HR calculation, proof read and commented on final draft
Michael Hallek (MH): clinical expertise, proof read and commented on final draft
Andreas Engert (AE): provided clinical expertise, proof read and commented on the first and final draft
Sources of support
Internal sources
-
Köln Fortune, Germany.
Funding programme “Köln Fortune”, Medical Faculty University of Cologne
External sources
-
BMBF, Germany.
Project grant application NO 01KG0918, Federal Ministry of Education and Research (BMBF)
Declarations of interest
NS, KB and IM: none known.
TE is a member of the German CLL Study Group and received honoraria and travel/accommodations/meeting expenses from Bayer Schering AG, Genzyme AG, and Mundipharma for presenting CLL‐related data
AE conducted several trials in this field. He received research funding and lecture fees from Bayer Schering and Genzyme AG.
VR did a four months paid internship at Novartis, working on a general statistical methods project as part of her doctoral training.
MH received lecture fees from Bayer AG and Genzyme AG and honoraria for consultancy from Bayer AG, Schering AG and Genzyme AG.
New
References
References to studies included in this review
CAM 307 {published data only}
- Demko S, Summers J, Keegan P, Pazdur R. FDA drug approval summary: alemtuzumab as single‐agent treatment for B‐cell chronic lymphocytic leukemia. Oncologist 2008;13:167‐74. [DOI] [PubMed] [Google Scholar]
- Dmoszynska A, Fetni R, Wang Y, Belkaci M, Sirard C, Goldberg M, et al. Cytogenetic correlation with efficacy on alemtuzumab (CAM) vs chlorambucil (CHLO) as front‐line therapy for patients with progressive B‐cell chronic lymphocytic leukemia (BCLL) [abstract]. Journal of Clinical Oncology 2006; Vol. 24:361.
- Genzyme. A phase III study to evaluate the efficacy and safety of front‐line therapy with alemtuzumab (Campath, MabCampath) vs chlorambucil in patients with progressive B‐cell chronic lymphocytic leukemia (ClinicalTrials.gov identifier: NCT00046683). http://www.clinicaltrials.gov 2003.
- Hillmen P, Skotnicki A, Robak T, Jaksic B, Dmoszynka A, Sirard C, et al. Preliminary phase III efficacy and safety of alemtuzumab vs chlorambucil as front‐line therapy for patients with progressive B‐cell chronic lymphocytic leukemia (BCLL) [abstract]. Journal of Clinical Oncology 2006; Vol. 24:339.
- Hillmen P, Skotnicki A, Robak T, Jaksic B, Sirard C, Mayer J. Alemtuzumab (Campath, MabCampath) Has superior progression free survival (PFS) vs chlorambucil as front‐line therapy for patients with progressive B‐cell chronic lymphocytic leukemia (BCLL) [Abstract]. Blood 2006; Vol. 108:93.
- Hillmen P, Skotnicki A, Robak T, Jaksic B, Sirard C, Mayer J. Progression free survival is superior with alemtuzumab vs chlorambucil as front‐line therapy for patients with B‐cell chronic lymphocytic leukemia [Abstract] 1996. Haematologica 2007;92:45‐6. [Google Scholar]
- Hillmen P, Skotnicki AB, Robak T, Jaksic B, Dmoszynska A, Wu J, et al. Alemtuzumab compared with chlorambucil as first‐line therapy for chronic lymphocytic leukemia. Journal of Clinical Oncology 2007;25:5616‐23. [DOI] [PubMed] [Google Scholar]
- Hillmen P, Skotnicki AB, Robak T, Mayer J, Jaksic B, Vukovic V, et al. Preliminary safety and efficacy report of a randomized trial of alemtuzumab vs chlorambucil as front‐line therapy in 297 patients with progressive B‐cell chronic lymphocytic leukemia. Blood 2004; Vol. 104:687a.
- Hillmen PH, Skotnicki AS, Robak TR, Jaksic BJ, Dmoszynska AD, Sirard CS, et al. Preliminary results for the phase III trial of alemtuzumab (Campath, MabCampath) vs chlorambucil as first‐line treatment for B‐cell chronic lymphocytic leukemia (B‐cll) [Abstract]. Haematologica 2006;91:381. [Google Scholar]
- Mayer J, Robak T, Skotnicki A, Jaksic B, Dmoszynka A, Sirard C, et al. Impact of prognostic factors on outcome in a phase III study comparing alemtuzumab to chlorambucil as first‐line therapy for B‐cell chronic lymphocytic leukemia (BCLL) [abstract]. Journal of Clinical Oncology 2007; Vol. 25:365. [DOI] [PubMed]
- Mayer J, Robak T, Skotnicki A, Jaksic B, Vukovic V, Weitman S. Interim safety summary of alemtuzumab (Campath, Mabcampath) vs chlorambucil as front‐line therapy for patients with progressive B‐cell chronic lymphocytic leukemia [abstract]. Hematology Journal 2004;5:28. [Google Scholar]
- Robak T, Dmoszynska A, Fetni R, Wang Y, Belkacz M, Sirard C. Incidence of genomic aberrations and associated efficacy from a phase III study comparing alemtuzumab (Campath, MabCampath) vs chlorambucil as first line therapy for B‐cell chronic lymphocytic leukemia (BCLL). Blood 2006; Vol. 108.
- Robak T, Skotnicki A, Dmoszynka A, Hebert J, Wang Y, Belkaci M, et al. Cytogenic abnormalities and associated efficacy from a phase iii study comparing alemtuzumab vs. Chlorambucil as first line therapy for b‐cell chronic leukemia [Abstract] 1997. Haematologica 2007;92:46. [Google Scholar]
- Robak T, Skotnicki AB, Mayer J, Vukovic V, Weitman S. Interim safety summary of alemtuzumab vs. chlorambucil as front‐line therapy for patients with progressive B‐cell chronic lymphocytic leukemia (BCLL) [abstract]. Journal of Clinical Oncology 2004; Vol. 22:571.
- Skotnicki AB, Robak T, Mayer J, Craig A, Weitman S. Phase III study to evaluate the efficacy and safety of front‐line therapy with alemtuzumab (Campath, MabCampath) vs. chlorambucil in patients with progressive B‐cell chronic lymphocytic leukemia (CAM307). Blood 2003;102:439a. [Google Scholar]
CAM 314 {published data only}
- Elter T, Gercheva‐Kyuchukova L, Pylylpenko H, Robak T, Jaksic B, Rekhtman G, et al. Fludarabine plus alemtuzumab versus fludarabine alone in patients with previously treated chronic lymphocytic leukaemia: a randomised phase 3 trial. The Lancet Oncology published online 2011 October 10 [Epub ahead of print]. [PUBMED: 21992852] [DOI] [PubMed]
- Engert A, Gercheva L, Pilipenko G, Robak T, Wu J, Barry A, et al. Fludarabine (Flu) plus alemtuzumab (FluCam) improves progression free survival versus fludarabine in previously treated chronic lymphocytic leukemia and demonstrates activity in high risk patients. Haematologica 2010;95:Suppl 2. [Google Scholar]
- Engert A, Gercheva L, Pilipenko G, Robak T, Wu J, Sirard CA, et al. Overall survival advantage and acceptable safety profile with fludarabine in combination with alemtuzumab (FluCam) in previously treated patients with advanced stage chronic lymphocytic leukemia. Blood 2010;116:919. [Google Scholar]
- Engert A, Gercheva L, Robak T, Galina P, Wu J, Sirard CA, et al. Improved progression‐free survival (PFS) of alemtuzumab (Campath, MabCampath) plus fludarabine (Fludara) versus fludarabine alone as second‐line treatment of patients with B‐cell chronic lymphocytic leukemia: preliminary results from a phase III randomized trial. Blood 2009;114:537. [Google Scholar]
CLL2007FMP {published data only}
- Lepretre S, Aurran T, Mahe B, Cazin B, Tournihlac O, Maisonneuve H, et al. Immunochemotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) versus fludarabine (F), cyclophosphamide (C) and MabCampath (Cam) (FCCam) in previously untreated patients (pts) with advanced B‐chronic lymphocytic leukemia (B‐CLL) : experience on safety and efficacy within a randomised multicenter phase III trial of the French Cooperative Group on CLL and WM (FCGCLL/MW) and the "Groupe Ouest‐Est d'Etudes Des Leucemies Aigues Et Autres Maladies Du Sang" (GOELAMS) : CLL2007FMP (for fit medically patients). Blood 2009;114:538. [Google Scholar]
- Remi L, Lepretre S, Arnoulet C, Baseggio L, Campos L, Chatelain B, et al. CLL2007FMP, a phase III randomized multicentric trial of the French Cooperative Group on CLL and WM (FCGCLL/MW) and the "Groupe Ouest‐Est D'etudes Des Leucemies Aigues Et Autres Maladies Du Sang" (GOELAMS): immunochemotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) yields a significantly better response than fludarabine (F), cyclophosphamide (C) and MabCampath (Cam) (FCCam) in previously untreated B‐chronic lymphocytic leukemia patients as evaluated by a sensitive 6 color flow cytometry MRD. Blood 2010;116:698. [Google Scholar]
GCLLSG CLL4B {published data only}
- Ritgen M, Schweighofer C, Fingerle‐Rowson G, Eichhorst B, Busch R, Stilgenbauer S. Consolidation with alemtuzumab in first remission induces pronounced MRD reduction and clinical remissions update on a randomized phase III trial of the German CLL Study Group (GCLLSG). Blood 2004; Vol. 104:687a. [DOI] [PubMed]
- Schweighofer C, Ritgen M, Eichhorst B, Busch R, Kneba M, Hallek M, et al. Consolidation with alemtuzumab improves progression‐free survival in patients with chronic lymphocytic leukemia (CLL) in first remission long‐term follow‐up of a randomized phase III trial of the German CLL Study Group (GCLLSG) [abstract]. Blood 2006;108:14. [DOI] [PubMed] [Google Scholar]
- Schweighofer CD, Ritgen M, Eichhorst BF, Busch R, Abenhardt W, Kneba M, et al. Consolidation with alemtuzumab improves progression‐free survival in patients with chronic lymphocytic leukaemia (CLL) in first remission: long‐term follow‐up of a randomized phase III trial of the German CLL Study Group (GCLLSG). British Journal of Haematology 2009;144:95‐8. [DOI] [PubMed] [Google Scholar]
- Schweighofer CD, Ritgen M, Fingerle‐Rowson G, Eichhorst B, Busch R, Stilgenbauer S, et al. Consolidation with alemtuzumab in patients with chronic lymphocytic leukemia (CLL) in first remission ‐ update on a randomized phase III trial of the German CLL Study Group (GSLLSG). Onkologie 2004;27:28‐9. [DOI] [PubMed] [Google Scholar]
- Wendtner CM, Ritgen M, Fingerle‐Rowson G, Jaeger G, Campe H, Engert A, et al. High efficacy but considerable toxicity of campath‐1H consolidation therapy in CLL patients responding to fludarabine ‐ results of a randomized phase III trial [abstract]. Journal of Clinical Oncology 2003;22:579. [Google Scholar]
- Wendtner CM, Ritgen M, Schweighofer CD, Fingerle‐Rowson G, Campe H, Jaeger G. Consolidiation with alemtuzumab in patients with chronic lymphocytic leukemia (CLL) in first remission ‐ experience on safety and efficacy within a randomized multicenter phase III trial of the German CLL Study Group (GCLLSG). Blood 2003;102:676. [DOI] [PubMed] [Google Scholar]
- Wendtner CM, Ritgen M, Schweighofer CD, Fingerle‐Rowson G, Campe H, Jager G, et al. Consolidation with alemtuzumab in patients with chronic lymphocytic leukemia (CLL) in first remission‐‐experience on safety and efficacy within a randomized multicenter phase III trial of the German CLL Study Group (GCLLSG). Leukemia 2004;18:1093‐101. [DOI] [PubMed] [Google Scholar]
- Wendtner CM, Ritgen M, Schweighofer CD, Fingerle‐Rowson G, Campe H, Jager G, et al. High efficacy but considerable toxicity of Alemtuzumab (MabCampath) consolidation therapy in CLL patients responding to Fludarabine or Fludarabine/cyclophosphamide ‐ results of a randomized phase III trial of the GCLLSG. Onkologie 2003;26:173. [Google Scholar]
- Wendtner CM, Ritgen M, Schweighofer CD, Fingerle‐Rowson G, Campe H, Jager G, et al. High efficacy but considerable toxicity of alemtuzumab (Campath‐1H) consolidation therapy in CLL patients responding to fludarabine ‐ results of a randomized phase III trial of the GCLLSG [abstract: 0610]. Haematologica 2003;88:Suppl. [Google Scholar]
Gribben 2005 {published data only}
- Gribben J, Stephans K, Blossom M. Fludarabine and alemtuzumab versus fludarabine and rituximab in the treatment of relapsed B‐cell chronic lymphocytic leukemia: Results from a phase II study [abstract]. Blood 2005;106:343. [Google Scholar]
References to studies excluded from this review
Bolli 2004 {published data only}
- Bolli N, Ianni M, Simonetti S, Cerroni L, Liso A, Falini B, et al. Treating two concurrent B‐cell and T‐cell lymphoid neoplasms with alemtuzumab monotherapy. Lancet Oncology 2004;5:64‐5. [DOI] [PubMed] [Google Scholar]
Byrd 2009 {published data only}
- Byrd JC, Peterson BL, Rai KR, Hurd D, Hohl R, Perry MC, et al. Fludarabine followed by alemtuzumab consolidation for previously untreated chronic lymphocytic leukemia: final report of Cancer and Leukemia Group B study 19901. Leukemia & Lymphoma 2009;50:1589‐96. [DOI] [PubMed] [Google Scholar]
Elter 2009 {published data only}
- Elter T, Kilp J, Borchmann P, Schulz H, Hallek M, Engert A. Pharmacokinetics of alemtuzumab in combination with fludarabine in patients with relapsed or refractory B‐cell chronic lymphocytic leukemia. Haematologica 2009;94:150‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faderl 2010 {published data only}
- Faderl S, Ferrajoli A, Wierda W, O'Brien S, Lerner S, Keating M J. Alemtuzumab by continuous intravenous infusion followed by subcutaneous injection plus rituximab in the treatment of patients with chronic lymphocytic leukemia recurrence.[Erratum appears in Cancer. 2010 Aug 15;116(16):3982 Note: Dosage error in article text]. Cancer 2010;116:2360‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hale 2004 {published data only}
- Hale G, Rebello P, Brettman LR, Fegan C, Kennedy B, Kimby E, et al. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood 2004;104:948‐55. [DOI] [PubMed] [Google Scholar]
Karlsson 2006 {published data only}
- Karlsson C, Norin S, Kimby E, Sander B, Porwit Macdonald A, Nilsson B, et al. Alemtuzumab as first‐line therapy for B‐cell chronic lymphocytic leukemia: long‐term follow‐up of clinical effects, infectious complications and risk of Richter transformation. Leukemia 2006;20:2204‐7. [DOI] [PubMed] [Google Scholar]
Kennedy 2000 {published data only}
- Kennedy B, Forsyth PD, Smith GM, Rawstrom AC, Byrne J, Haynes A, et al. Campath ‐ 1h therapy does not affect pbsc collection and engraftment in patients with CLL previously treated with fludarabine [abstract]. Hematology Journal 2000;1:201. [Google Scholar]
Lin 2010 {published data only}
- Lin TS, Donohue KA, Byrd FC, Lucas MS, Hoke EE, Bengtson EM, et al. Consolidation therapy with subcutaneous alemtuzumab after fludarabine and rituximab induction therapy for previously untreated chronic lymphocytic leukemia: final analysis of CALGB 10101. Journal of Clinical Oncology 2010;28:4500‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osterborg 1996 {published data only}
- Osterborg A, Fassas AS, Anagnostopoulos A, Dyer MJ, Catovsky D, Mellstedt H. Humanized CD52 monoclonal antibody Campath‐1H as first‐line treatment in chronic lymphocytic leukaemia. British Journal of Haematology 1996;93:151‐3. [DOI] [PubMed] [Google Scholar]
Pettitt 2006 {published data only}
- Pettitt AR, Matutes E, Oscier D. Alemtuzumab in combination with high‐dose methylprednisolone is a logical, feasible and highly active therapeutic regimen in chronic lymphocytic leukaemia patients with p53 defects. Leukemia 2006;20:1441‐5. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
2006‐0767 {published data only}
- Alemtuzumab + rituximab consolidation in CLL (NCT00771602). Ongoing study August 2008.
39338 {published data only}
- Low dose alemtuzumab for consolidation and maintenance of patients with B‐cell CLL (NCT00336206). Ongoing study June 12, 2006.
CAM203 {published data only}
- Subcutaneous alemtuzumab (CAMPATH®, MabCampath®) in relapsed/refractory B‐CLL. Ongoing study May 18, 2006.
CLL8 {published data only}
- CLL8: a randomised, phase III study to assess alemtuzumab consolidation therapy in patients with CLL who have responded to previous therapy (ISRCTN63375144). Ongoing study June 2008.
DMS‐F0334 {published data only}
- Fludarabine combined with either alemtuzumab or rituximab in treating patients with refractory or relapsed B‐CLL (NCT00086775). Ongoing study July 2003.
Hovon 68 CLL {published data only}
- A randomised phase III study in previously untreated patients with biological high‐risk CLL: fludarabine and cyclophosphamide (FC) versus FC and low‐dose alemtuzumab (ISRCTN25180151). Ongoing study December 2005.
P04715 {published data only}
- SCH 727965 in patients with mantle cell lymphoma or B‐CLL (study P04715AM2) (NCT00871546). Ongoing study March 2009.
Additional references
Binet 1981
- Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 1981;48(1):198‐206. [DOI] [PubMed] [Google Scholar]
Byrd 2009a
- Byrd JC, Peterson BL, Rai KR, Hurd D, Hohl R, Perry MC, et al. Fludarabine followed by alemtuzumab consolidation for previously untreated chronic lymphocytic leukemia: final report of Cancer and Leukemia Group B study 19901. Leukemia and Lymphoma 2009;50:1589‐96. [DOI] [PubMed] [Google Scholar]
Chiorazzi 2005
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. The New England Journal of Medicine 2005;352(8):804‐15. [DOI] [PubMed] [Google Scholar]
Coles 2011
- Coles AJ, Fox E, Vladic A, Gazda SK, Brinar V, Selmaj KW, et al. Alemtuzumab versus interferon beta‐1a in early relapsing‐remitting multiple sclerosis: post‐hoc and subset analyses of clinical efficacy outcomes. Lancet Neurology 2011;10(4):338‐48. [DOI] [PubMed] [Google Scholar]
Cortelezzi 2009
- Cortelezzi A, Pasquini MC, Gardellini A, Gianelli U, Bossi A, Reda G, et al. Low‐dose subcutaneous alemtuzumab in refractory chronic lymphocytic leukaemia (CLL): results of a prospective, single‐arm multicentre study. Leukemia 2009;23:2027‐33. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Dohner 2000
- Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. The New England Journal of Medicine 2000;343(26):1910‐6. [DOI] [PubMed] [Google Scholar]
Eichhorst 2006
- Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecher C, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first‐line therapy of younger patients with chronic lymphocytic leukemia. Blood 2006;107(3):885‐91. [DOI] [PubMed] [Google Scholar]
Elter 2005
- Elter T, Borchmann P, Schulz H, Reiser M, Trelle S, Schnell R, et al. Fludarabine in combination with alemtuzumab is effective and feasible in patients with relapsed or refractory B‐cell chronic lymphocytic leukemia: results of a phase II trial. Journal of Clinical Oncology 2005;23(28):7024‐31. [DOI] [PubMed] [Google Scholar]
Elter 2009a
- Elter T, Vehreschild JJ, Gribben J, Cornely OA, Engert A, Hallek M. Management of infections in patients with chronic lymphocytic leukemia treated with alemtuzumab. Annals of Hematology 2009;88:121‐32. [DOI] [PubMed] [Google Scholar]
Flinn 2007
- Flinn IW, Neuberg DS, Grever MR, Dewald GW, Bennett JM, Paietta EM, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. Journal of Clinical Oncology 2007;25(7):793‐8. [DOI] [PubMed] [Google Scholar]
Flynn 2007
- Flynn JM, Byrd JC. Have we forgotten the purpose of phase III studies?. Journal of Clinical Oncology 2007;25(35):5553‐5. [DOI] [PubMed] [Google Scholar]
Fraser 2006
- Fraser G, Smith CA, Imrie K, Meyer R, Hematology Disease Site Group. Alemtuzumab in chronic lymphocytic leukemia: a clinical practice guideline. Program in Evidence‐based Care. Evidence‐based series. Vol. 6‐16, Toronto (ON): Cancer Care Ontario (CCO): AHRQ ‐ Agency for Healthcare Research + Quality, 2006. [Google Scholar]
GRADEpro [Computer program]
- Jan B, Andrew O, Holger S. GRADEpro. Version 3.2 for Windows. Cochrane IMS, 2008.
Hainsworth 2008
- Hainsworth JD, Vazquez ER, Spigel DR, Raefsky E, Bearden JD, Saez RA, et al. Combination therapy with fludarabine and rituximab followed by alemtuzumab in the first‐line treatment of patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase 2 trial of the Minnie Pearl Cancer Research Network. Cancer 2008;112:1288‐95. [DOI] [PubMed] [Google Scholar]
Hallek 2008
- Hallek M, Cheson BD, Catovsky D, Caligaris‐Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute‐Working Group 1996 guidelines. Blood 2008;111(12):5446‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallek 2008a
- Hallek M, Fingerle‐Rowson G, Fink A, Busch, R, Mayer J, Hensel M, et al. Immunochemotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) versus fludarabine and cyclophosphamide (FC) improves response rates and progression‐free survival (PFS) of previously untreated patients (pts) with advanced chronic lymphocytic leukemia (CLL). ASH Conference Proceedings. 2008:abstract 325.
Higgins 2011
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (March 2011). Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Hillmen 2007
- Hillmen P, Skotnicki AB, Robak T, Jaksic B, Dmoszynska A, Wu J, et al. Alemtuzumab compared with chlorambucil as first‐line therapy for chronic lymphocytic leukemia. Journal of Clinical Oncology 2007;25(35):5616‐23. [DOI] [PubMed] [Google Scholar]
Kaufman 2011
- Kaufman MS, Caramanica A, Janson D, Driscoll N, Johnson C, Kohn N, et al. Alemtuzumab maintenance may safely prolong chemotherapy‐free intervals in chronic lymphocytic leukemia. Med Oncol 2011;28:532‐8. [DOI] [PubMed] [Google Scholar]
Keating 2002
- Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J, et al. Therapeutic role of alemtuzumab (Campath‐1H) in patients who have failed fludarabine: results of a large international study. Blood 2002;99(10):3554‐61. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [DOI] [PubMed] [Google Scholar]
Moreton 2005
- Moreton P, Kennedy B, Lucas G, Leach M, Rassam SM, Haynes A, et al. Eradication of minimal residual disease in B‐cell chronic lymphocytic leukemia after alemtuzumab therapy is associated with prolonged survival. Journal of Clinical Oncology 2005;23(13):2971‐9. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Rai 1975
- Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood 1975;46(2):219‐34. [DOI] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Schünemann 2011
- Schnemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings tables'. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Steurer 2006
- Steurer M, Pall G, Richards S, Schwarzer G, Bohlius J, Greil R. Purine antagonists for chronic lymphocytic leukaemia. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004270.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stilgenbauer 2009
- Stilgenbauer S, Zenz, T, Winkler D, Buhler A, Schlenk R, Groner S, et al. Subcutaneous alemtuzumab in fludarabine‐refractory chronic lymphocytic leukemia: clinical results and prognostic marker analyses from the CLL2H study of the German Chronic Lymphocytic Leukemia Study Group. Journal of Clinical Oncology 2009;27(24):3994‐4001. [DOI] [PubMed] [Google Scholar]
Thursky 2006
- Thursky KA, Worth LJ, Seymour JF, Miles PH, Slavin MA. Spectrum of infection, risk and recommendations for prophylaxis and screening among patients with lymphoproliferative disorders treated with alemtuzumab*. British Journal of Haematology 2006;132(1):3‐12. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weingart 2009
- Weingart O, Elter T, Bauer K, Brillant C, Herbst C, Monsef I, et al. Monoclonal anti‐CD20 antibodies for chronic lymphocytic leukaemia. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008079] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wendtner 2004
- Wendtner CM, Ritgen M, Schweighofer CD, Fingerle‐Rowson G, Campe H, Jager G, et al. Consolidation with alemtuzumab in patients with chronic lymphocytic leukemia (CLL) in first remission‐‐experience on safety and efficacy within a randomized multicenter phase III trial of the German CLL Study Group (GCLLSG). Leukemia 2004;18(6):1093‐101. [DOI] [PubMed] [Google Scholar]
Wierda 2005
- Wierda WG, Kipps TJ, Keating MJ. Novel immune‐based treatment strategies for chronic lymphocytic leukemia. Journal of Clinical Oncology 2005;23(26):6325‐32. [DOI] [PubMed] [Google Scholar]
Wierda 2006
- Wierda W, O'Brien S, Faderl S, Ferrajoli A, Wang X, Do KA, et al. A retrospective comparison of three sequential groups of patients with recurrent/refractory chronic lymphocytic leukemia treated with fludarabine‐based regimens. Cancer 2006;106(2):337‐45. [DOI] [PubMed] [Google Scholar]
Wierda 2011
- Wierda WG, Kipps TJ, Keating MJ, Brown JR, Gribben JG, Browning M, et al. Self‐administered, subcutaneous alemtuzumab to treat residual disease in patients with chronic lymphocytic leukemia. Cancer 2011;117:116‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]


