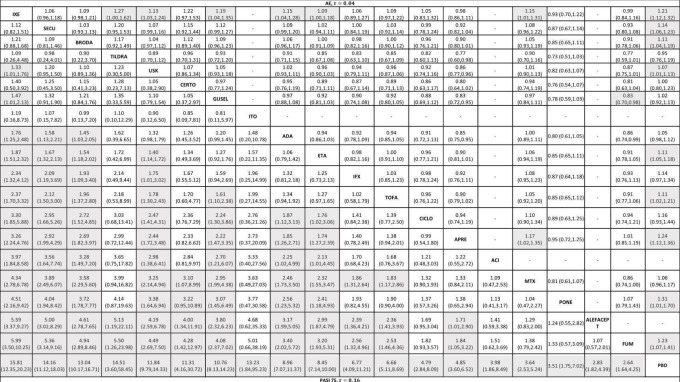
Figure 8.
Relative effects of the intervention as estimated from the network meta‐analysis model for Psoriasis Area and Severity Index (PASI 75) and adverse events (AEs)
Drugs are reported in order of primary benefit ranking. Each cell contains the Risk Ratio (RR) and 95% confidence interval for the two secondary outcomes (PASI 75 and adverse events) of the intervention in the respective column versus the comparator in the respective row. RRs larger than 1 for the lower triangle and smaller than 1 for the upper triangle favour the treatment on the left. Significant results are are highlighted in grey.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; τ (Tau): estimated heterogeneity standard deviation parameter; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab