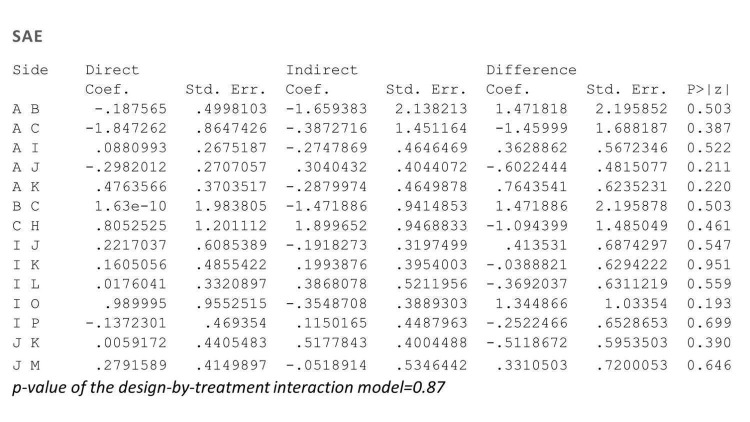
Figure 24.
Side‐splitting approach and design‐by‐treatment interaction model for inconsistency for serious adverse events (SAEs)
Treatment codes: A = PBO, B = FUM, C = MTX, D = ACI, E = ALEFACEPT, F = CICLO, G = IFX, H = ADA, I = ETA, J = USK, K = SECU, L = IXE, M = BRODA, N = CERTO, O = APRE, P = TOFA, Q = GUSEL, R = TILDRA, S = PONE, T = ITO
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab