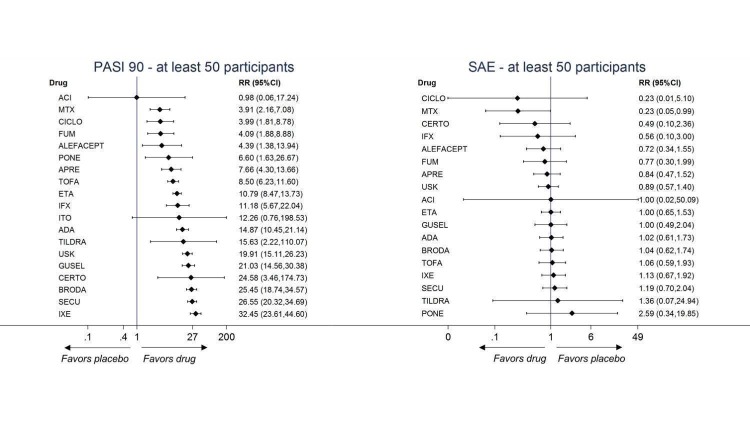
Figure 27.
Sensitivity analyses ‐ Interval plot. Network meta‐analysis results for primary outcomes (PASI 90 and serious adverse events) for trials with at least 50 participants.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
CI: confidence interval; PASI: Psoriasis Area and Severity Index; RR: risk ratio; SAE: serious adverse events