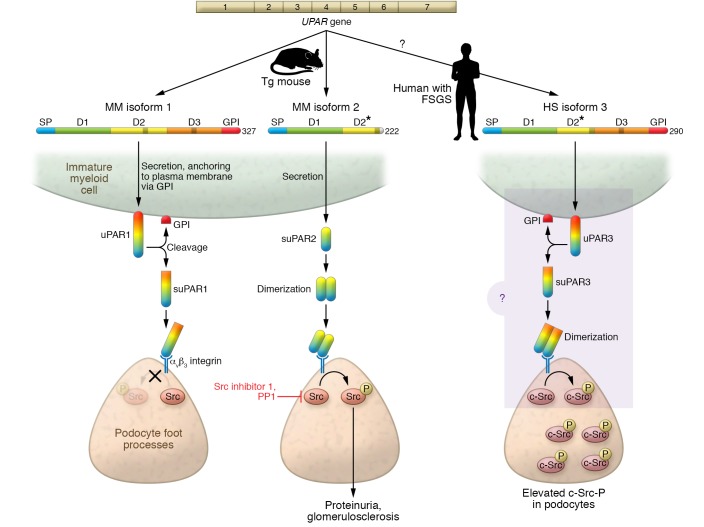
Figure 1. Mechanism of uPAR in podocyte injury.
Mus musculus (MM) uPAR1 isoform 1 is secreted and anchored to the plasma membrane via the GPI anchor, and is then converted from a membrane-bound form (uPAR) into a soluble form (suPAR) by enzymatic removal of the GPI anchor. MM uPAR isoform 2 lacks a GPI anchor and hence is a suPAR; in contrast to MM isoform 1, it forms a soluble dimer. In mice transgenic for either MM isoform 1 or MM isoform 2, suPAR binds avβ3 integrin located at the podocyte foot process plasma membrane. Binding of suPAR isoform 2, possibly as a dimer, generates phosphorylated c-Src, through outside-in signaling. By contrast, for isoform 1, no c-Src-P was detected. Transgenic mice bearing suPAR isoform 2 uniquely develop proteinuria and glomerulosclerosis. Targeting of c-Src with Src-specific inhibitors (Src inhibitor 1 or PP1) reduced proteinuria. Homo sapiens (HS) uPAR isoform 3 is the result of in-frame exon splicing leading to preservation of the GPI anchor and is predicted to form dimers, resembling MM isoform 2. Elevated levels of phosphorylated c-Src were found in human kidney biopsy samples, as with MM suPAR isoform 2 mice. This suggests that in human podocytes HS suPAR isoform 3 functions similarly to MM isoform 2, increasing phosphorylated c-Src, which drives the development of FSGS. D, domain. D2*, truncated D2.