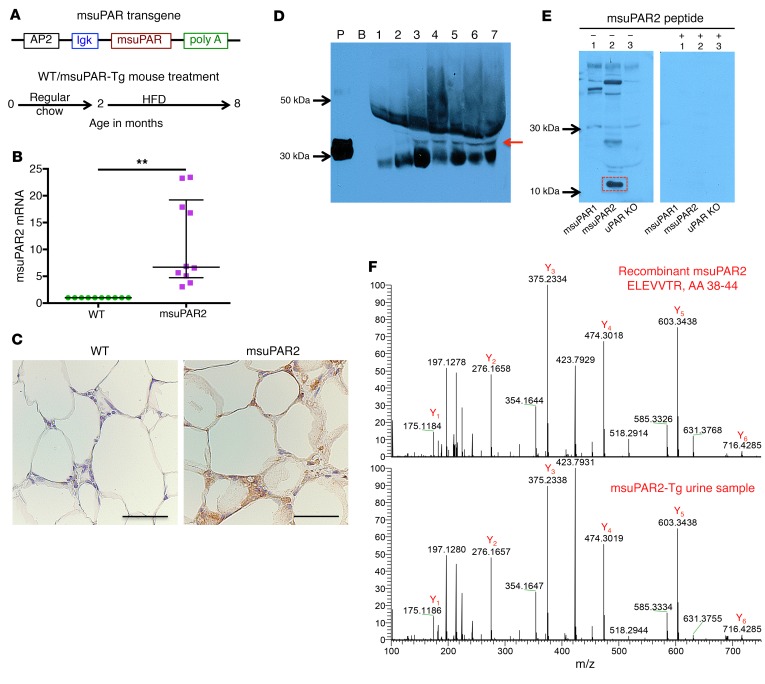
Figure 2. Detection of msuPAR2 in adipocytes, serum, and urine.
(A) Schematic of msuPAR2-Tg construction and msuPAR2-Tg mouse treatment. (B) qPCR analysis of muPAR2 in fat tissues. The value was calculated as a ratio of muPAR2 differential expression between littermate controls (WT) and msuPAR2-Tg mice over that of housekeeping gene GAPDH. muPAR2 mRNA was increased significantly compared with littermate controls. Mann-Whitney U test. **P < 0.01. (C) Localization of msuPAR2 in adipocytes. As msuPAR2 carries the c-Myc tag, immunohistochemistry was performed with a rabbit anti-Myc antibody. msuPAR2 was seen in adipocytes of msuPAR2-Tg mice, but not in littermate control mice. Scale bar: 50 μm. (D) Detection of msuPAR2 in circulating blood in msuPAR2-Tg mice. Albumin-depleted sera were separated by NuPAGE gel and processed for Western blot with rabbit anti-msuPAR2 antibody. P, recombinant msuPAR2 protein; B, blank without protein samples. Lane 1, uPAR KO sera; lane 2, msuPAR1-Tg sera; lanes 3 to 7, sera from different msuPAR2-Tg mice. Images shown are representatives of 3 different experiments. Red arrow indicates msuPAR2. (E) Detection of msuPAR2 in urine. Processed urine samples were separated by SDS-PAGE and blotted with a customized rabbit anti-msuPAR2 antibody. A band at 10–15 kDa (highlighted in red rectangle) was identified in msuPAR2-Tg but not in msuPAR1-Tg nor in uPAR-KO mice. Preincubation of the antibody with msuPAR2 peptide nullified the band. (F) Verification of msuPAR2 fragment by LC-MS analysis. The msuPAR2 fragment identified by Western blot was processed for MS analysis. Multiple peptides in the N-terminal region were detected. Shown is one of these peptides (bottom panel), which matches very well with the spectrum of the peptide from recombinant msuPAR2 protein (top panel).