Abstract
Context:
Quality of life is increasingly recognized as an important outcome of cancer treatment. Previous studies have examined clinical predictors of quality of life, but with the increasing prevalence of wearable sensors that monitor sleep and activity patterns, further investigation into whether these behaviors are predictive of post-treatment quality of life is now feasible. Among patients receiving aggressive cancer treatment such as hematopoietic cell transplantation (HCT), analysis of circadian rhythms (24-hour patterns of sleep and activity) via wearable sensors is limited.
Objective:
Evaluate the relationship between overall quality of life and circadian rhythms in patients receiving allogeneic HCT.
Methods:
Patients wore an ActiGraph GT3X (Pensacola, FL) activity monitor for at least 72 hours prior to the initiation of conditioning chemotherapy and transplantation, and completed a quality of life (FACT-G) assessment. Quality of life assessments were also completed 1, 3, and 6 months after HCT.
Results:
Patients (n=45, M age=55) were mostly male (66%) with a total FACT-G score of 80.96 (SD=16.05) pre-HCT. Mixed models revealed robust cross-sectional associations between overall quality of life and multiple circadian rhythmicity parameters, including durations of high physical activity, overall circadian rhythmicity, and earlier starts of daily activity (ps<.01). Recovery of quality of life post-transplant was predicted by longer pre-transplant durations of high physical activity (p=.04) and earlier evening retirement (p=.04).
Conclusion:
Our findings suggest that wearable sensor information is a promising method of predicting recovery of quality of life after HCT. Additional studies are needed to confirm these findings in a larger sample.
Keywords: quality of life, wearable devices, HCT, circadian rhythms
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative treatment for hematologic malignancies, with recent advances in HCT techniques improving transplant-related morbidity, mortality rates, and post-transplant relapse.1,2 In addition to medical outcomes, quality of life (QoL) has gained increasing attention as an important metric of HCT success for both patients and clinicians. Overall, long-term QoL remains impaired in HCT recipients relative to population norms.2,3 However, assessing average QoL may obscure heterogeneity in recovery patterns following transplant and may not reflect the varied recovery patterns in QoL as demonstrated by previous longitudinal assessments.4 Notably, some patients demonstrate preserved physical QoL at baseline that subsequently declines after HCT with no evidence of QoL recovery by six months post-transplant.4 Several factors, including older age, female sex, and advanced disease have previously been identified as at risk indicators for poor recovery of QoL.3 However, these sociodemographic and disease predictors do not fully explain QoL trajectories after HCT.4 Thus, novel approaches are needed to identify patients at risk for poor QoL recovery following transplant.
Wearable activity sensors, previously available for research purposes only, are now ubiquitously commercially available and can be used to evaluate patient behaviors prior to transplant. An actigraph, for example, is a small sensor typically worn on the wrist that uses an accelerometer to infer physical activity (i.e., activity), sleep, and wake cycles.5 Utilization of actigraphs allows for passive and non-invasive tracking of daily activity and sleep patterns in real time. One objective marker of behavioral patterns that can be derived from wearable sensors is circadian rhythmicity, or the consistent timing and magnitude of sleep and activity patterns that follow a daily 24-hour cycle. Circadian rhythmicity is conceptually distinct from separate assessments quantifying objective sleep and physical activity.6 Individuals with normal circadian patterns tend to be active during the day and inactive at night, with consistent objective sleep and activity patterns. In contrast, cancer patients show dysregulated circadian rhythms, often during cancer treatment,7 as evidenced by being less active during the day and exhibiting flattened variability in magnitude of daily rest and activity levels,8–10 and having inconsistent patterns of sleep and activity.9–11 In cancer patients, less robust circadian rhythms are associated with higher levels of systemic inflammation12 and an increased risk of disease progression and mortality.13 In patients with suspected cancer, less robust circadian rhythms may also predict tumor malignancy.14
Though limited, existing literature supports the association between QoL and circadian rhythmicity in cancer patients.15–19 Rhythmic 24-hour rest/activity periods are associated with higher concurrent QoL in addition to more favorable medical outcomes (e.g., treatment response, survival) in patients with various types of cancer, including breast, colorectal, and lung cancer.16,17,19–21 These associations have been observed over and above the impact of sex, age, primary tumor, number of metastatic sites, and prior treatment.17 However, to our knowledge, no studies have examined the predictive value of circadian rhythmicity for identifying recovery of quality of life. Further, circadian rhythms in HCT recipients have been largely unexplored. Thus, the goal of this pilot study was to evaluate the relationship between circadian rhythmicity in cancer patients prior to receiving allogeneic HCT and QoL throughout the transplant course. We hypothesized that increased baseline rhythmicity would be associated with better concurrent QoL prior to HCT and would predict faster recovery of QoL after HCT.
Methods
Eligible participants were: at least 18 years of age, scheduled to receive allogeneic HCT, had completed vital organ testing for allogeneic HCT, had a hemoglobin value of ≥ 8 g/dL, had an ECOG performance status of ≤ 2, and were able to walk unassisted. Written informed consent was obtained prior to initiation of study procedures. The study was approved by University of South Florida IRB. Following written informed consent, participants filled out a baseline questionnaire assessing quality of life. A research coordinator then attached an actigraph to the wrist of patients’ choosing and explained its use (after completion of vital organ testing and within 30 days of admission for allogeneic HCT). Seven days later, the actigraph was removed by study staff. Participants were asked to complete follow-up questionnaires at 1, 3, and 6 months after transplant.
Measures and Materials
Demographic and clinical information.
Patient-related variables obtained through medical record review included: date of birth, comorbidities, gender, donor source, and primary diagnosis. Comorbidities were calculated using the Hematopoietic Cell Transplantation Comorbidities Index (HCT-CI).22 Higher scores on the HCT-CI reflect greater comorbidity (e.g., cardiovascular disease, infection).
Circadian rhythmicity.
Daily activity patterns were evaluated using GT3X wrist actigraphs (Actigraph LLC, Pensacola, FL). Actigraphs have been shown to be valid and reliable measures of circadian patterns5,21,23 and feasible for patients receiving autologous or allogeneic HCT.24–26 Each actigraph uses a three-axis accelerometer to assess and record physical movement, which is then averaged over every minute. To maintain consistency across different durations of actigraphic monitoring, and consistent with evidence-based practice parameters,23 raw continuous accelerometer data from the first 72 hours of consecutive wear time (starting at 12:00 AM) were exported into a comma separated value (.csv) worksheet using Actilife 6.13.3 (Actigraph LLC, Pensacola, FL). Time and vector magnitude were then input into a five-parameter extended cosine model with an antilogistic transformation of the standard cosine curve27 in SAS 9.4 to derive circadian parameters. Selected representative statistics reported here include: width ratio, up-mesor, down-mesor, and f-statistic (Supplemental Table 1). The width ratio characterizes the duration of high activity compared to low activity, with larger numbers indicating longer durations of high activity. The up-mesor refers to the time of day when participants became active, with larger numbers indicating a later daily start of activity. The down-mesor refers to the time of day when participants’ activity declined for the evening, with larger numbers indicating a later daily decline of activity. The f-statistic describes “the strength and endurance of a [circadian] rhythm, as a measure of the signal-to-noise ratio,”28 calculated as “the percentage of the variance accounted for by the fitted cosine model”28 accounting for the number of observations and parameters in the model.27 A larger F-statistic indicates a more robust circadian rhythm.29
Quality of life.
The Functional Assessment of Cancer Therapy-General (FACT-G),30 plus a Bone Marrow Transplant-specific subscale (not reported here) was used to assess QoL before HCT, and 1, 3, and 6 months after HCT. The FACT-G has been used extensively in HCT recipients. In addition to a total score, this scale includes four subscales: physical (PWB; 7 items), social (SWB; 7 items), emotional (EWB; 6 items), and functional well-being (FWB; 7 items). In this study, we evaluated the total FACT-G score as the primary outcome. Additional probing of subscales was conducted if the omnibus tests of total FACT-G were significant. Higher scores indicated better QoL.
Data Analysis
Data from participants who wore the actigraph for at least 72 hours were included in final analyses.23 Means and standard deviations (continuous variables) and frequencies and percentages (categorical variables) were used to describe participant characteristics. Mixed models (i.e., PROC MIXED) were used to evaluate cross-sectional and longitudinal relationships between baseline circadian rhythmicity and the total FACT-G score over time, which allows for the use of all available data without having to impute missing data31 and controls for outcomes at baseline (pre-transplant). To aid interpretation, circadian predictors were mean-centered, and width ratio was rescaled from 0 to 1 to 0–10 and f-statistic was divided by 100. Circadian rhythmicity was evaluated by including the interactions between circadian rhythmicity and time after controlling for potential covariates (age, comorbidities), main effects, and other two-way interactions. Covariates were chosen a priori and included factors that have been shown by previous literature4 to be associated with physical QoL. Consistent with previous literature suggesting a nonlinear relationship between time and QoL,4 mixed models included the quadratic effect of time. To reduce the potential for Type 1 error, further probing of QoL was conducted if the omnibus tests of total FACT-G were significant. The effect size of the predictors was computed using pseudo-R2, 31 All analyses were conducted in SAS Version 9.4 (Cary, NC). All tests were two-sided and alpha was set at p<0.05.
Results
Fifty patients signed consent, but three did not receive HCT and were excluded from analyses. Of the remaining 47 patients, two had less than 72 hours of continuous accelerometer data and were excluded from analyses. The final sample consisted of 45 patients. Participant characteristics are shown in Table 1. Sociodemographic factors (age, comorbidities, gender) were not associated with total FACT-G prior to transplant (ps>.18). Consistent with previous literature, age and comorbidity were included as covariates.3
Table 1.
Participant Characteristics (N=45)
| Variable | Mean (SD) / Median (range) / N (%) |
|---|---|
| Age: mean (SD) | 54.8 (13.8) |
| Comorbidities: median (range) | 3 (0–8) |
| Female: frequency (%) | 15 (33.3) |
| Donor Source: frequency (%) | |
| Umbilical cord blood | 1 (2.2) |
| MMUD | 10 (22.2) |
| MUD | 21 (46.7) |
| MRD | 13 (28.9) |
| Primary Diagnosis: frequency (%) | |
| AML | 10 (22.2) |
| MDS | 8 (17.8) |
| ALL | 5 (11.1) |
| NHL, B-Cell | 4 (8.9) |
| NHL, T/NK-Cell | 4 (8.9) |
| HD | 3 (6.7) |
| MM | 3 (6.7) |
| Other | 8 (17.7) |
Note: MMUD = mismatched unrelated donor, MUD = matched unrelated donor, MRD = matched related donor, AML = acute myeloid leukemia, MDS = myelodysplastic syndrome, ALL = acute lymphocytic leukemia, NHL = Non-Hodgkin’s Lymphoma, HD = Huntington’s disease, MM = multiple myeloma
Prior to HCT, total FACT-G averaged 80.96 (SD=16.05). Participants’ scores dropped to 70.90 (SD=14.70) by one month post-transplant. At three months post-transplant, total FACT-G rebounded to 79.77 (SD=13.82), and by six months post-transplant scores were stable at 82.08 (SD=13.63). On average, participants became active (up-mesor) at 7:55 a.m. (95% CI: 7:19 to 8:31 a.m., range: 5:00 a.m. to 4:56 p.m.). Activity peaked (mean acrophase) at 3:25 p.m. (95% CI: 2:57 to 3:52 p.m., range: 12:31 to 8:00 p.m.). On average, participants’ activity declined for the evening (down-mesor) at 10:55 p.m. (95% CI: 10:19 to 11:30 p.m., range: 6:12 p.m. to 4:29 a.m.).
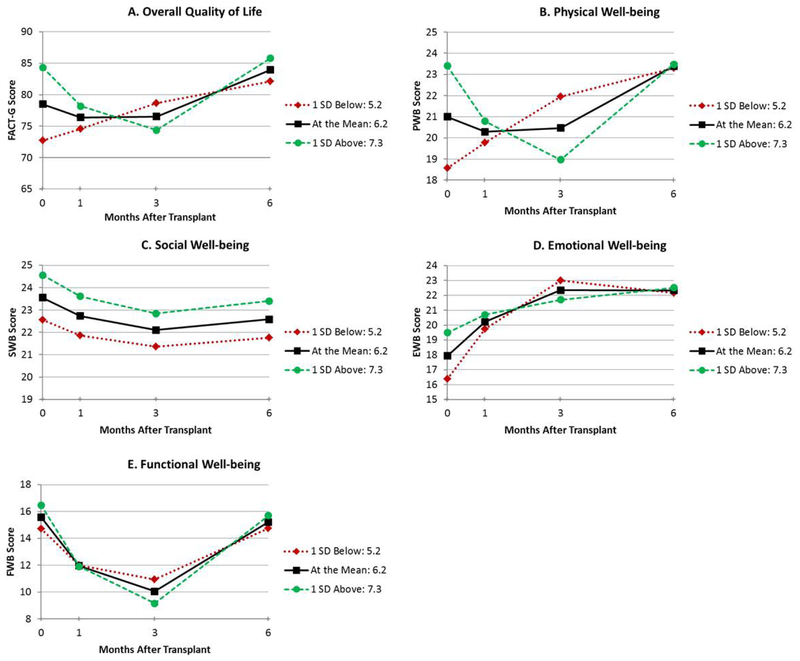
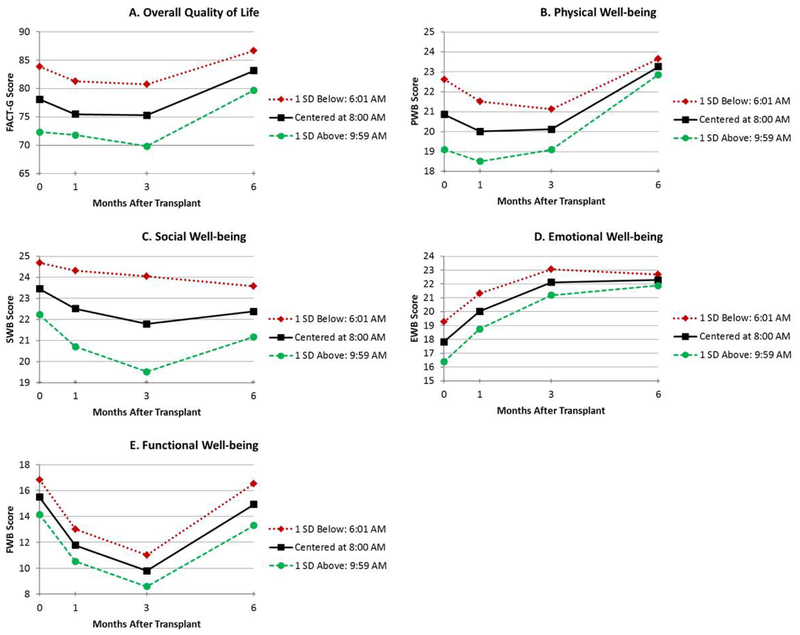
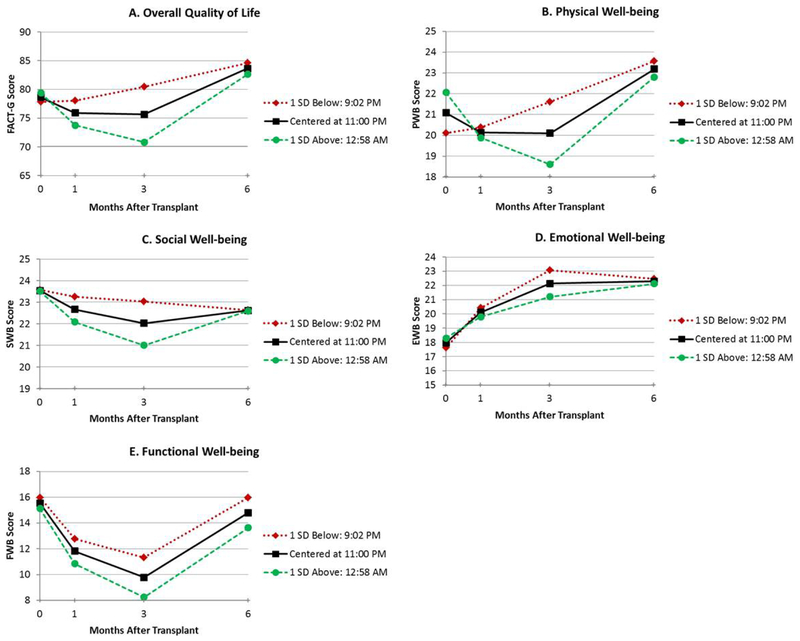
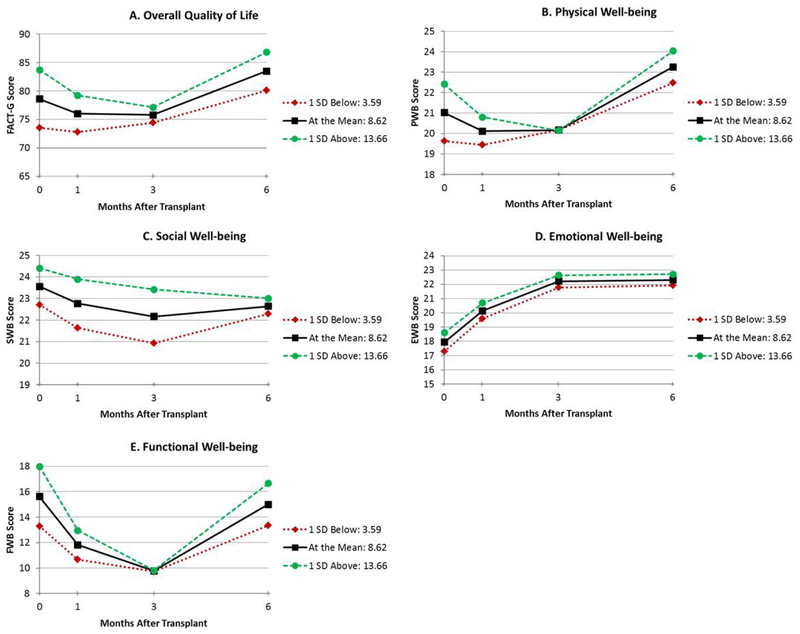
The results of the mixed models adjusted for age and comorbidities are shown in Table 2. Figures depicting unadjusted QoL over time by circadian predictor are presented for duration of high activity (width ratio; Figure 1A–E), the time at which participants became active (up-mesor; Figure 2A–E), the time at which participants’ activity declined in the evening (down-mesor; Figure 3A–E), and overall circadian rhythmicity (f-statistic; Figure 4A–E). Overall, circadian rhythmicity parameters accounted for 3–22% of the variance in the intercept of total FACT-G. Prior to receiving HCT, patients with longer durations of high activity (width ratio) reported better concurrent total FACT-G (p=0.02) (Figure 1A), physical well-being (p=0.005) (Figure 1B), and emotional well-being (p=0.01) (Figure 1D). Patients who became active earlier in the day (up-mesor) reported better concurrent total FACT-G (p=0.05) (Figure 2A) and emotional well-being (p=0.02) (Figure 2D). Patients with more rhythmic daily patterns of activity (f-statistic) reported better concurrent total FACT-G (p=0.01) (Figure 4A) and functional well-being (p<0.01) (Figure 4E). There were no significant associations between the time at which patients’ activity declined in the evening and concurrent total FACT-G (ps>0.06). Thus, patients who were more active pre-HCT, started their day earlier, and had typical, consistent patterns of sleep and activity had better concurrent QoL.
Table 2.
Unstandardized Mixed Model Parameter (Beta) Estimates
| Circadian Variable | Effect | Total QOL | PWB | SWB | EWB | FWB |
|---|---|---|---|---|---|---|
| Width ratio | Intercept | 78.53*** | 20.97*** | 23.56*** | 17.93*** | 15.59*** |
| Time | −1.76 | −0.59 | −0.56 | 1.29** | −2.39*** | |
| Time*Time | 0.47* | 0.17 | 0.09 | −0.16* | 0.47*** | |
| Width ratio | 4.66* | 2.13** | 0.68 | 1.41* | 0.63 | |
| Time*Width ratio | −4.38* | −1.99* | −0.14 | −1.21* | −1.02 | |
| Time*Time*Width ratio | 0.63* | 0.27* | 0.01 | 0.17* | 0.16 | |
| Up-mesor | Intercept | 78.40*** | 20.94*** | 23.54*** | 17.88*** | 15.55*** |
| Time | −2.00 | −0.70 | −0.60 | 1.23** | −2.41*** | |
| Time*Time | 0.51* | 0.19 | 0.10 | −0.15* | 0.47*** | |
| Up-mesor | −2.30* | −0.73 | −0.40 | −0.78* | −0.58 | |
| Time*up-mesor | 0.21 | −0.05 | −0.26 | 0.17 | 0.24 | |
| Time*Time*up-mesor | <.01 | 0.03 | 0.06 | −0.01 | −0.04 | |
| Down-mesor | Intercept | 78.65*** | 21.04*** | 23.56*** | 17.96*** | 15.58*** |
| Time | −1.92 | −0.65 | −0.57 | 1.24** | −2.41*** | |
| Time*Time | 0.50* | 0.18 | 0.10 | −0.15* | 0.47*** | |
| Down-mesor | 1.09 | 0.76 | 0.14 | 0.27 | −0.11 | |
| Time*down-mesor | −1.82* | −0.94* | −0.26 | −0.42 | −0.27 | |
| Time*Time*down-mesor | 0.27* | 0.13* | 0.05 | 0.06 | 0.04 | |
| F-statistic | Intercept | 78.62*** | 21.00*** | 23.56*** | 17.95*** | 15.62*** |
| Time | −2.08 | −0.71 | −0.54 | 1.22** | −2.50*** | |
| Time*Time | 0.52* | 0.19 | 0.09 | −0.15* | 0.49*** | |
| F-statistic | 1.04** | 0.27 | 0.19 | 0.13 | 0.48** | |
| Time*F-statistic | −0.47 | −0.17 | 0.06 | −0.02 | −0.30* | |
| Time*Time*F-statistic | 0.07 | 0.02 | −0.01 | 0.00 | 0.05* |
Note: QoL = quality of life, PWB = physical well-being, SWB = social well-being, EWB = emotional well-being, FWB = functional well-being. Models adjusted for mean-centered age and comorbidities and each covariate*time and covariate*time*time interaction. All outcomes are mean-centered. Width ratio is multiplied by 10 and f-statistic is divided by 100 for ease of interpretation. The intercept represents the value of the outcome pre-transplant and the circadian variable effect evaluates whether the circadian rhythmicity parameter was associated with the outcome pre-transplant. Longitudinal changes are modeled as time and time*time effects, and the associations between each circadian rhythmicity parameter and time are examined using the effect*time and effect*time*time interaction, which account for baseline QoL.
p < .001,
p < .01,
p < .05
Figure 1.
Unadjusted estimated means for duration of high activity compared to low activity (width ratio) (≤ 1 SD below mean, mean, and ≥1 SD above mean) by total QoL (FACT-G; A), physical well-being (PWB; B), social well-being (SWB; C), emotional well-being (EWB; D), and functional well-being (FWB; E)
Figure 2.
Unadjusted estimated means for the time of day at which participants became active (up-mesor) (≤ 1 SD below mean, mean, and ≥1 SD above mean) by total QoL (A), physical well-being (B), social well-being (C), emotional well-being (D), and functional well-being (E)
Figure 3.
Unadjusted estimated means for the time of day at which participants retired for the evening (down-mesor) (≤ 1 SD below mean, mean, and ≥1 SD above mean) by total QoL (A), physical well-being (B), social well-being (C), emotional well-being (D), and functional well-being (E)
Figure 4.
Unadjusted estimated means for overall circadian rhythmicity (f-statistic) (≤ 1 SD below mean, mean, and ≥1 SD above mean) by total QoL (A), physical well-being (B), social well-being (C), emotional well-being (D), and functional well-being (E)
Throughout the transplant course, patients with longer durations of high physical activity (width ratio) pre-HCT had greater recovery of total FACT-G (p=0.02) (Figure 1A), physical well-being (linear p=0.02, quadratic p=0.05) (Figure 1B) and emotional well-being (linear p=0.02, quadratic p=0.04) (Figure 1D). Patients whose activity declined earlier in the evening (down-mesor) pre-HCT had greater recovery of total FACT-G (linear p=0.03, quadratic p=0.05) (Figure 3A), physical well-being (linear p=0.01, quadratic p=0.03) (Figure 3B). There were no significant associations between the time at which patients became active in the morning and recovery of total FACT-G (ps>0.11). Thus, patients who were more active and whose activity declined earlier in the evening pre-HCT had better QoL throughout the transplant course.
Discussion
To our knowledge, this pilot study is the first to examine the relationship between circadian rhythmicity and QoL in patients receiving allogeneic HCT. Results indicate that circadian rhythmicity data from wearable sensors can predict recovery of QoL after HCT. There was a robust pattern of cross-sectional associations such that prior to transplantation, better QoL was associated with being active earlier in the morning, increased daily activity, and greater overall circadian rhythmicity. These findings extend previous research identifying associations between QoL and circadian patterns of rest and activity in other cancer populations, including patients with breast cancer,15 metastatic colorectal cancer,16,17,19 and advanced lung cancer.18
Previously examined sociodemographic and clinical predictors of post-transplant recovery of QoL have evidenced only moderate effectiveness.32 Our data show that recovery of QoL after allogeneic HCT was predicted by longer durations of high activity and earlier declines in evening activity prior to transplantation. It is unclear why “early birds” might have better QoL throughout the transplant course, but these findings are consistent with cross-sectional findings in women with breast cancer prior to receiving chemotherapy.15 Further, our results suggest that the timing of daily activity—not just the quantity—is important for post-transplant outcomes. Prior to receiving HCT, patients had similar levels of QoL but different concurrent and longitudinal patterns of circadian activity. Thus, circadian rhythmicity may not be a proxy for QoL, but rather a useful predictor of QoL, particularly for patients receiving allogeneic HCT who may be especially likely to experience impairments in QoL.3,33
There are several approaches for modifying sleep and wake patterns to improve regulation of circadian rhythmicity. Suggested interventions for future evaluation might include cognitive-behavioral approaches tailored to sleep, wake, and activity patterns. For example, cognitive-behavioral therapy for insomnia involves “sleep restriction,” a practice in which a patient anchors her wake time at a certain hour each morning and adjusts her bedtime accordingly to encourage efficient sleep. This therapy could be combined with gradual, consistent exercise (as acceptable medically), activity pacing, and daily scheduling to better manage sleep-wake patterns. Other interventions, such as bright light therapy34 or melatonin administration,35 may also improve QoL recovery patterns after HCT in at-risk patients. In addition, preventive rehabilitation, or “prehab,” may be effective at modifying patients’ circadian patterns of activity prior to receiving HCT. From a mechanistic perspective, Costanzo and colleagues36 suggest that behavioral factors (e.g., circadian patterns of rest and activity) in patients receiving HCT may directly influence immune recovery and subsequent quality of life. Thus, modifying circadian patterns of activity may be especially efficacious in transplant recipients.
This longitudinal report is the first to our knowledge that includes a focused evaluation of circadian rhythmicity as a predictor of post-HCT quality of life utilizing an innovative data collection method. While our study demonstrates the predictive value of circadian rhythmicity on transplant recovery, limitations include a single assessment of circadian rhythmicity pre-HCT in a small sample that received heterogeneous HCT treatment protocols, and no corrections for multiple comparisons. In addition, these transplant patients had very disordered circadian rhythms (e.g., wide ranges for the circadian parameters of interest). Nevertheless, this study’s findings advance our understanding of circadian patterns of activity as a correlate and predictor of quality of life in patients receiving HCT.
In summary, research on predictors of QoL recovery is needed. Passive data collection strategies, which limit patient burden, offer objective measurements of circadian rhythmicity that may predict recovery of QoL. These approaches are particularly important for patients receiving HCT due to high rates of morbidity. In this study, predictive patterns of circadian rhythmicity were identified in under 72 hours prior to HCT. Clinically, replication of our results would suggest that use of passively collected data from wearable sensors to establish circadian patterns prior to treatment is feasible and clinically meaningful for predicting QoL recovery. With the increasing availability of commercial wearable fitness trackers (e.g., FitBit), additional examination of the clinical efficacy of wearable sensors is warranted. Future research would benefit from confirming these results utilizing a larger cohort with circadian rhythmicity assessments before and after treatment.
Supplementary Material
Acknowledgment:
This study was funded by the Moffitt Cancer Center Bone Marrow Transplant Foundation and the National Cancer Institute (R25-CA090314). We thank Dr. Sonia Ancoli-Israel for providing assistance with data interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Norkin M, Wingard JR. Recent advances in hematopoietic stem cell transplantation. F1000Res. 2017;6:870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pidala J, Anasetti C, Jim H. Health-related quality of life following haematopoietic cell transplantation: Patient education, evaluation and intervention. Br J Haematol. 2010;148(3):373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jim HSL, Sutton SK, Small BJ, et al. Trajectories of quality of life after hematopoietic cell transplantation: Secondary analysis of Blood and Marrow Transplant Clinical Trials Network 0902 data. Biol Blood Marrow Transplant. 2016;22(11):2077–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 6.Chen HM, Wu YC, Tsai CM, Tzeng JI, Lin CC. Relationships of circadian rhythms and physical activity with objective sleep parameters in lung cancer patients. Cancer Nurs. 2015;38(3):215–223. [DOI] [PubMed] [Google Scholar]
- 7.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(6):971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevalier V, Mormont MC, Cure H, Chollet P. Assessment of circadian rhythms by actimetry in healthy subjects and patients with advanced colorectal cancer. Oncol Rep. 2003;10(3):733–737. [PubMed] [Google Scholar]
- 9.Liu L, Rissling M, Neikrug A, et al. Fatigue and circadian activity rhythms in breast cancer patients before and after chemotherapy: A controlled study. Fatigue. 2013;1(1–2):12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pati AK, Parganiha A, Kar A, Soni R, Roy S, Choudhary V. Alterations of the characteristics of the circadian rest-activity rhythm of cancer in-patients. Chronobiol Int. 2007;24(6):1179–1197. [DOI] [PubMed] [Google Scholar]
- 11.Ancoli-Israel S, Liu L, Rissling M, et al. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: A 1-year longitudinal study. Support Care Cancer. 2014;22(9):2535–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rich T, Innominato PF, Boerner J, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11(5):1757–1764. [DOI] [PubMed] [Google Scholar]
- 13.Levi F, Dugue PA, Innominato P, et al. Wrist actimetry circadian rhythm as a robust predictor of colorectal cancer patients survival. Chronobiol Int. 2014;31(8):891–900. [DOI] [PubMed] [Google Scholar]
- 14.Hoogland AI, Bulls HW, Gonzalez BD, et al. Differential patterns of circadian rhythmicity in women with malignant versus benign gynecologic tumors. Psychooncology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14(3):201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mormont MC, Waterhouse J, Bleuzen P, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res. 2000;6(8):3038–3045. [PubMed] [Google Scholar]
- 17.Mormont MC, Waterhouse J. Contribution of the rest-activity circadian rhythm to quality of life in cancer patients. Chronobiol Int. 2002;19(1):313–323. [DOI] [PubMed] [Google Scholar]
- 18.Grutsch JF, Ferrans C, Wood PA, et al. The association of quality of life with potentially remediable disruptions of circadian sleep/activity rhythms in patients with advanced lung cancer. BMC Cancer. 2011;11:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Innominato PF, Focan C, Gorlia T, et al. Circadian rhythm in rest and activity: A biological correlate of quality of life and a predictor of survival in patients with metastatic colorectal cancer. Cancer Res. 2009;69(11):4700–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ancoli-Israel S, Moore PJ, Jones V. The relationship between fatigue and sleep in cancer patients: A review. European journal of cancer care. 2001;10(4):245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grutsch JF, Wood PA, Du-Quiton J, et al. Validation of actigraphy to assess circadian organization and sleep quality in patients with advanced lung cancer. J Circadian Rhythms. 2011;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: An update for 2002. Sleep. 2003;26(3):337–341. [DOI] [PubMed] [Google Scholar]
- 24.Nelson AM, Jim HSL, Small BJ, et al. Sleep disruption among cancer patients following autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2018;53(3):307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson AM, Coe CL, Juckett MB, et al. Sleep quality following hematopoietic stem cell transplantation: Longitudinal trajectories and biobehavioral correlates. Bone Marrow Transplant. 2014;49(11):1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Jurdi N, Cotton JM, Ali N, et al. Use of a fitness tracking device to monitor physical activity and sleep patterns during hospitalization for hematopoietic stem cell transplant: Results of a prospective observational study. Biology of Blood and Marrow Transplantation. 2018;24(3):S258–S259. [Google Scholar]
- 27.Marler MR, Gehrman P, Martin JL, Ancoli-Israel S. The sigmoidally transformed cosine curve: A mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med. 2006;25(22):3893–3904. [DOI] [PubMed] [Google Scholar]
- 28.Refinetti R, Lissen GC, Halberg F. Procedures for numerical analysis of circadian rhythms. Biological rhythm research. 2007;38(4):275–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Rissling M, Neikrug A, et al. Fatigue and Circadian Activity Rhythms in Breast Cancer Patients Before and After Chemotherapy: A Controlled Study. Fatigue : biomedicine, health & behavior. 2013;1(1–2):12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. [DOI] [PubMed] [Google Scholar]
- 31.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford university press; 2003. [Google Scholar]
- 32.Braamse AM, Yi JC, Visser OJ, et al. Developing a risk prediction model for long-term physical and psychological functioning after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(3):549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong FL, Francisco L, Togawa K, et al. Long-term recovery after hematopoietic cell transplantation: predictors of quality-of-life concerns. Blood. 2010;115(12):2508–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neikrug AB, Rissling M, Trofimenko V, et al. Bright light therapy protects women from circadian rhythm desynchronization during chemotherapy for breast cancer. Behav Sleep Med. 2012;10(3):202–216. [DOI] [PubMed] [Google Scholar]
- 35.Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin. 2010;5(4):701–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costanzo ES, Juckett MB, Coe CL. Biobehavioral influences on recovery following hematopoietic stem cell transplantation. Brain, Behavior, and Immunity. 2013;30 Suppl(Suppl):S68–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.