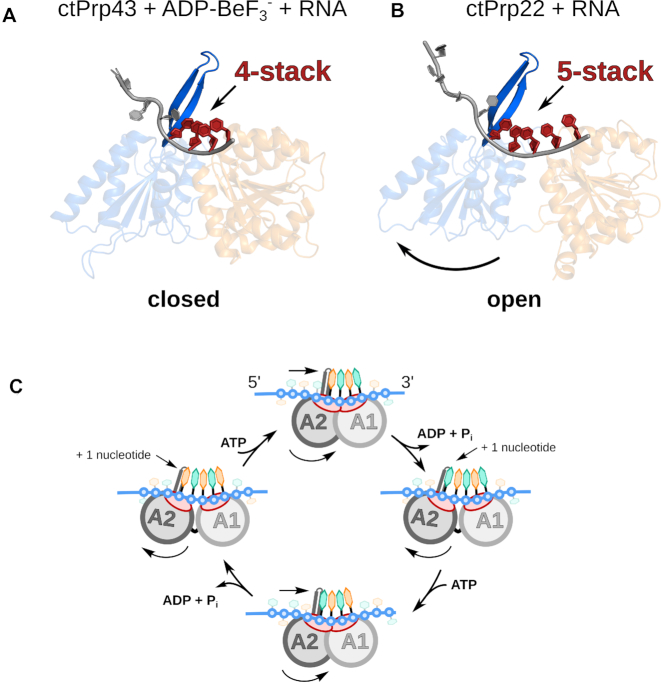
Figure 4.
DEAH-box ATPases translocate at a step-size of one RNA nucleotide per hydrolyzed ATP. The helicase cores of ctPrp43 and ctPrp22 with bound ssRNAs are depicted as cartoon models with a domain coloring according to Figure 1. (A) ATP-bound ctPrp43 exhibits a closed conformation of the helicase core with a stack of four RNA nucleotides bound to the RecA-like domains. (B) In the absence of an adenosine nucleotide the helicase core of ctPrp22 adopts an open conformation, which allows the accommodation of an additional RNA nucleotide in the binding tunnel leading to a bound five-nucleotide stack. In both conformations, the stack is interrupted by the β-hairpin of the RecA2 domain. (C) A continuous cycling of these states enables DEAH-box ATPases to translocate in 3′ to 5′ direction along a single-stranded RNA. Upon helicase core opening, an additional RNA nucleotide can be incorporated between the β-hairpin and the first RNA nucleotide of the four-nucleotide stack. The helicase core closure induced by the binding of ATP pushes the RNA through the binding tunnel.