Figure 1.
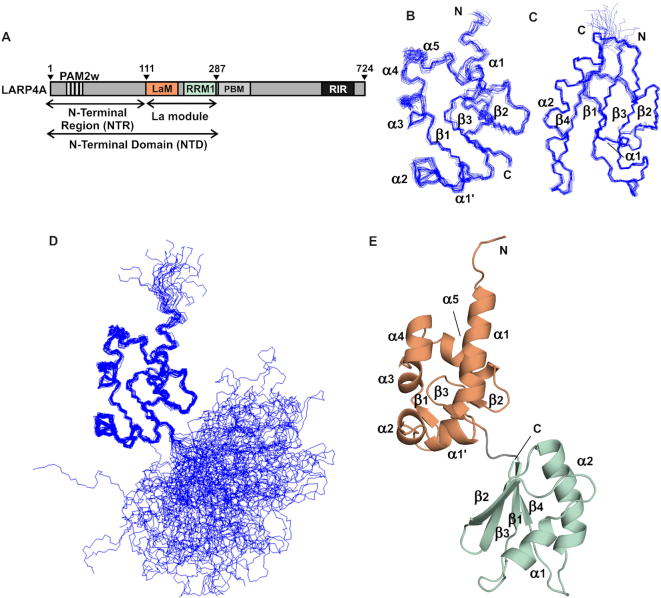
Domain organization of human LARP4A and solution structure of the La module. (A) Domain architecture of human LARP4A and nomenclature of fragments used in this study. The NTD spans residues 1–287 and the NTR residues 1–111. LARP4A La module, encompassing amino acids 111–287, is composed of two domains: the LaM (111–196) and the RRM1 (200–287). The PAM2w motif (13-26) and the PBM (287–358) are the PABP-binding sites, and RIR is the RACK1 interaction region. (B–D) Backbone traces of the 20 lowest energy structures of the La module, superposed separately on (B) the LaM (showing aa 118–196) and (C) the RRM1 (showing aa 199–275). (D) View of the entire La module superposed on LaM highlighting the non-fixed relative orientation of the two domains in solution. (E) Representative structure of LARP4A La module in cartoon representation. The LaM and the RRM1 are coloured as in panel (A). The N and C-termini, α-helices and β-strands are indicated.