Abstract
Inflammation associated with autoimmune diseases and chronic injury is an initiating event that leads to tissue degeneration and dysfunction. Inflammatory monocytes and neutrophils systemically circulate and enter inflamed tissue, and pharmaceutical based targeting of these cells has not substantially improved outcomes and have had side effects. Herein, we investigated the design of drug-free biodegradable nanoparticles, notably without any active pharmaceutical ingredient or targeting ligand, that target circulating inflammatory monocytes and neutrophils in the vasculature to inhibit them from migrating into inflamed tissue. Nanoparticles were formed from 50:50 poly(DL-lactide-co-glycolide) (PLG) with two molecular weights (Low, High) and poly(DL-lactide) (PLA) formed (termed PLG-L, PLG-H, and PDLA, respectively) and were analyzed for their association with monocytes and neutrophils and their impact on disease course along with immune cell trafficking,. For particles injected intravenously for 6 consecutive days to mice with experimental autoimmune encephalomyelitis (EAE), PLG-H particles had significantly lower EAE clinical scores than PBS control, while PLG-L and PDLA particles had modest or negligible effect on EAE onset. In vivo and in vitro data suggests that PLG-H particles had high association with immune cells, with preferential association with blood neutrophils relative to other particles. PLG-H particles restrained immune cells from the central nervous system (CNS), with increased accumulation in the spleen, which was not observed for mice receiving PDLA or control treatments. These results demonstrate that the particle composition influences the association with inflammatory monocytes and neutrophils in the vasculature, with the potential to redirect trafficking and ameliorate inflammation.
Keywords: monocytes, neutrophils, inflammation, nanomedicine, poly(lactide-co-glycolide) nanoparticles
1. Introduction
Inflammation following infection or trauma is mediated by leukocytes and often results in exacerbation of a variety of immunological disorders. The cells responsible for response to an inflammatory injury typically originate in the bone marrow as inflammatory or classical monocytes that are then recruited to the injured or infected sites where they mature into macrophages and dendritic cells [1]. Here they produce chemical mediators activating the local vasculature to promote inflammation, as well as mediating other associated function. Similarly, neutrophils are recruited to inflamed tissue as the first line of defense and function alongside monocytes, macrophage and dendritic cells as part of this myeloid lineage response [2–4]. While this process represents a natural response to a stimulus, excessive cellular infiltration is pathologic and can be observed in a wide range of disease including viral or bacterial infections, traumatic brain or spinal cord injury, stroke, and autoimmune diseases such as multiple sclerosis (MS) [2,5–7].
Numerous options have been investigated in treatments for inflammatory disorders from generalized suppression through the delivery of antibodies, proteins, peptides, nucleic acid, and fluid infusion [8–10]. The use of broadly immunosuppressive treatments, such as corticosteroid, is accompanied by significant side effects, including muscle weakness, osteoporosis, diabetes, and neurodegeneration [11]. More specific monoclonal antibodies, such as natalizumab, have been used to prevent mononuclear leukocytes migration across the blood-brain barrier (BBB) into the brain parenchyma, while Rituximab has been used to deplete B-cells. However, in spite of the improved specificity of their suppression, these and similar treatments still may have incapacitating side effects, such as an increased incidence of opportunistic infections, including progressive multifunctional leukoencephalopathy [12,13]. Through efforts to avoid some of these complications and improve outcomes, delivering nanoparticles to target sites of inflammation has recently been identified as a promising alternative approach [14]. Nanoparticles carrying proteins and peptides have been used to target macrophages, dendritic cell and B-cells to suppress inflammation [15–18]. In addition, nanoparticles have emerged target immune cells and may be loaded with siRNA or other pharmaceuticals to influence the cellular response at the site [19,20]. Despite some highly promising results, the experimental and regulatory complexity of loading drugs and maintaining their stability, either on the particle surface or within them, has slowed their translation into clinical settings [21,22].
We have previously demonstrated that daily intravenous infusions of negatively-charged particles without any active pharmaceutical ingredient or targeting ligand (i.e., drug-free), that directly target inflammatory cells in the circulation reduced severity of acute stage inflammation in multiple mouse disease models, including West Nile virus encephalitis, sodium thioglycolate-induced peritoneal inflammation, experimental autoimmune encephalomyelitis (EAE), and spinal cord injury models [23,24]. In addition to particles without any drug, the uniqueness of the technique is the particles were intravenously injected to modulate immune cells in the blood compared with some of current treatments such as injecting nanoparticles loaded with anti-inflammatory drug or siRNA to local inflammatory sites [25,26]. Intravenously delivered nanoparticles associated with inflammatory monocytes in the blood, altering the degree of phosphatidylserine expression on their surface and redirecting the cells away from the inflamed sites and to the spleen. Similarly, neutrophils that associated with polystyrene particles were sequestrated within the liver, resulting in a reduction of neutrophils at the inflamed sites in an acute lung injury model [27]. Importantly, the reduction of clinical disease scores by particles treatment appears to be limited to the time over which particles are injected, suggesting that the treatment dose not deplete monocyte or neutrophil populations that underlie innate immunity. In previous studies, the negatively charged surfaces were a consistent design parameter across a range of materials including degradable poly(lactide-co-glycolide) (PLG), non-degradable polystyrene and diamond particles, which has been shown to influence scavenger receptor binding with relevant inflammatory cells [28]. These previous studies have demonstrated daily infusion of particles can ameliorate inflammation and biological mechanisms of varieties of disease models. However, the design of these biodegradable particles has not been studied, despite the potential for the different polymers to differentially impact immune cell function such as cellular association and internalization [29].
We investigated the design of PLG-based nanoparticles for their ability to attenuate inflammatory disease by associating with circulating immune cells in the blood and altering their phenotype (e.g., trafficking). PLG-based particles have been employed as drug carriers for immunomodulation with FDA approval and can be tuned their properties with varying ratio of lactide and/or glycolide and their molecular weight [30,31]. Regardless these benefits of PLG-based particles, effects of drug-free PLG-based particles on immunomodulation have not been fully investigated. We hypothesized that the designs of drug-free PLG-based particles can be tuned to target monocyte and neutrophil responses and influence their phenotypes resulting in amelioration of inflammation. Nanoparticles were formed from 50:50PLG with a low or high molecular weight (termed PLG-L and PLG-H, respectively), or poly (DL-lactide) (PLA) with a low molecular weight (termed PDLA), and were delivered intravenously into EAE mice for 6 consecutive days, during the initial phase of inflammatory monocyte and neutrophil migration into the CNS [32]. The internalization of particles by inflammatory monocytes (referred as Ly6Chi monocytes), non-classical monocytes (referred as Ly6Clow monocytes) and neutrophils were assayed in vitro and in vivo. We identified the trafficking and population dynamics of these cell types following particle administration, as well as the ability to influence EAE disease course. Collectively, these studies demonstrate that polymer composition and molecular weight substantially impact the trafficking and phenotype of inflammatory monocytes and neutrophils, which can be employed to ameliorate inflammation.
2. Materials and Methods
2.1. Materials
PLG-L (50:50 Poly(DL-lactide-co-glycolide), IV=0.17dL/g), PLG-H (50:50 Poly(DL-lactide-co-glycolide), IV=0.66dL/g), PDLA (Poly(DL-lactide), IV=0.18dL/g) with carboxylic acid end groups were purchased from DURECT corporation (Cupertino, CA). Poly(ethylene-alt-maleic anhydride) (PEMA) was purchased from Polyscience, Inc. (Warrington, PA). Proteolipid protein (HSLGKWLGHPDKF) (PLP139–151) was purchased from Peptide Synthesis Core at Northwestern University (Chicago, IL). Cyanine 5.5 amine dye was purchased from Lumiprobe (Florida, USA). Dichloromethane (DCM) and N-(3-Dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC) was purchased from Sigma-Aldrich (St. Louis, MO). N-hydroxysuccinimide (NHS) was purchased from Thermo Fisher Scientific (Waltham, MA).
All fluorochrome-conjugated antibodies and Fc block were purchased from BioLegend (San Diego, CA) as shown their names and catalog numbers here: TruStain FcX™ (Cat#101320, anti-mouse CD16/32) antibody, anti-mouse/human CD11b (Cat#101216, PE/Cy7, Clone: M1/70), anti-mouse CD45 (Cat#103136, PerCP, Clone: 30-F11), anti-mouse Ly-6C (Cat#128005, FITC, Clone: HK1.4), anti-mouse Ly6-G (Cat#127607, PE, Clone: 1A8), anti-mouse CD11c (Cat#117334, Brilliant Violet 605™, Clone: N418), anti-mouse CD4 (Cat#100510, FITC, Clone: RM4–5), and anti-mouse CD3 (Cat#100237, Brilliant Violet 605™, Clone: 17A2). LIVE/DEAD™ Fixable Violet Dead Cell Stain Kit, for 405 nm excitation, (Cat#L34964) was purchased from Life Technologies (Carlsbad, CA). Dilution was followed the manufactures’ instructions.
2.2. Mice
Female SJL/J mice were purchased from Envigo. All mice were housed under specific pathogen-free conditions and maintained in the University of Michigan in compliance with University Committee on Use and Care of Animal (UCUCA) regulations.
2.3. Particle Fabrication and Characterization
Conjugates of polymer and cyanine 5.5 amine dyes (Polymer-Cy5.5) were fabricated using a EDC/NHS chemistry [33]. Polymer (0.002 mmol) was dissolved in 5 mL of DCM in a 20-mL scintillation vial and mixed with 1.7 mg of EDC in 1 mL DCM for 5 min. NHS (1.0mg) in 0.5mL of DCM was added dropwise to the mixture and allowed to stir for 10 min. Finally, the 1.5mg of cyanine 5.5 amine dye in 1mL DCM was added to the mixture and stirred overnight. The solution was purified using 3500 molecular weight cut-off dialysis membrane in 4 L of distilled water over 3 days.
Particles containing Polymer-Cy5.5 were fabricated using the o/w single emulsion solvent evaporation method [34]. Briefly, 200mg of polymer containing 1% (w/v) of Polymer-Cy5.5 dissolved in dichloromethane was added to 1% (w/v) PEMA solution (PLG-L) or 2% (w/v) PEMA solution (PLG-H and PDLA), and sonicated using a Cole-Parmer CPX130 Ultrasonic Processor with a Cole-Parmer 3mm probe with stepped tip (Cole-Parmer Inc., Vernon Hills, IL). The emulsion was poured into 0.5%(w/v) PEMA solution and stirred on a Bellstir Multistir magnetic stirrer (Bellco Glass Inc., Vinland, NJ) overnight to remove organic phase. The resulting polymeric particles were washed three times and lyophilized with 2% (w/v) sucrose and 3% (w/v) D-mannitol. Nanoparticle morphology was analyzed using Tescan RISE scanning electron microscopy (SEM) after particles were coated with gold. Particle size and surface ζ-potential distributions in water were obtained using dynamic light scattering on a Zetasizer Nano ZSP (Malvern Instruments Ltd, Malvern, United Kingdom).
2.4. In Vitro particle uptake by monocytes and neutrophils
Peripheral blood was collected from EAE mice 9 days after immunization, and red blood cells were lysed using ACK (Ammonium-Chloride-Potassium) lysing buffer. 100,000 cells were seeded to each well of a 24-well plate (not-treated polystyrene), and cultured in 500μL of RPMI 1640 supplemented with 10% of fetal bovine serum (FBS), 1% penicillin/streptomycin and L-glutamine. 20 and 200μg/mL of PLG-H, PLG-L and PDLA particles were added to each well, and incubated for 0.5, 1 and 3 hours. At each time point, the cells were harvested, suspended in PBS and incubated with Fc Block and live dead staining for 15 minutes. Then, the cells were subsequently incubated with fluorochrome-conjugated antibodies (anti-mouse Ly-6C, anti-mouse Ly-6G, anti-mouse/human CD11b, anti-mouse CD45). Single stain, fluorescence-minus-one (FMO) stain and full panel stains were performed. Data was acquired with a Moflo Astrios (Beckman Coulter, Inc.) and analyzed with FlowJo software (FlowJo, LLC, Williamson Way, OR).
2.5. Induction of EAE and particle injection
Peptide-induced EAE was induced in SJL/J mice as previously reported [35,36]. 8–10 weeks old female mice were immunized subcutaneously at three spots on the flank with 100μL of an emulsion of PLP peptide in CFA containing 200 μg Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI). After 8 days of the disease immunization, particles (1.5 mg/day) suspended in PBS were intravenously injected through the tail for 6 days. PBS was also injected as a control.
2.6. Clinical Evaluation of Peptide-Induced EAE
Individual mice were observed daily, and EAE clinical scores were assessed on a 0–5 scale as follows: 0, no overt signs of disease; 1, limp tail or hind limb weakness but not both; 2, limp tail and hind limb weakness; 3, partial limb paralysis; 4, complete limb paralysis; 5, moribund state. The data are reported as the mean daily clinical score. Paralyzed mice were given easier access to food and water.
2.7. Evaluation of in vivo particle destination
Animals are euthanized at 14 days post immunization (p.i.), optical images of brain, spinal cord, liver and spleen was taken using an IVIS Lumina LET camera system (Caliper Life Sciences, Hopkinton, MA). Near-infrared fluorescence (NIR) images were obtained with cyanine 5.5 filter channel (emission: 720 nm, excitation: 675 nm).
2.8. Cell isolation and Flow cytometry
Single cells from blood and spleen were obtained as follows: blood was collected in EDTA-coated tubes and lysed using ACK lysing buffer, and then the rest of the cells were passed through 40-μm nylon mesh: Splenocytes were passed through a 70-μm nylon and 40-μm nylon mesh filter. All cells were treated with ACK lysing buffer and washed with PBS. Brain and spinal cord were harvested and ground, and single cells were isolated on a discontinuous percoll gradient.
Isolated cells were suspended in PBS and Fc Block and incubated for 15 minutes and subsequently incubated with fluorochrome-conjugated antibodies (anti-mouse Ly-6C, anti-mouse Ly-6G, antimouse/human CD11b, anti-mouse CD45, anti-mouse CD3, and anti-mouse CD4) besides live dead staining. Single stain, fluorescence-minus-one (FMO) stain and full panel stains were performed. Data was acquired with a Moflo Astrios (Beckman Coulter, Inc.) and analyzed with FlowJo software.
2.9. In Vivo particle uptake by monocytes and neutrophils
PLG-H and PDLA particles were injected to 10 days p.i. EAE mice. Peripheral blood was collected in EDTA-coated tubes at 1 and 3 hours after particles injection. Red blood cells were lysed using ACK buffer, and the rest of cells were passed through 40-μm nylon mesh. The isolated cells were blocked and stained with anti-mouse Ly-6C, Ly-6G, CD11b, and CD45 and Live Dead for flow cytometry analysis as the previously mentioned.
2.10. Statistical Analysis
GraphPad Prism software (San Diego, CA) was employed for all statistical analysis. Normality was assessed by Shapiro-Wilk test. Two-way analysis of variance (ANOVA) followed by Tukey was used for in vivo EAE studies over the time. One-way ANOVA followed by Tukey or nonparametric analysis followed by Dunn’s test for comparison of three or more groups or t-test using Holm-Sidak method for comparison of two groups was performed in the other in vivo and in vitro studies. Data represented as mean ± standard deviation (SD). Asterisks indicate p values of * < 0.05, ** < 0.01, and *** < 0.001 to show significant difference between the groups unless noted otherwise.
3. Results
3.1. Preparation and characterization of three types of nanoparticles with 500nm diameter and highly negative-charge
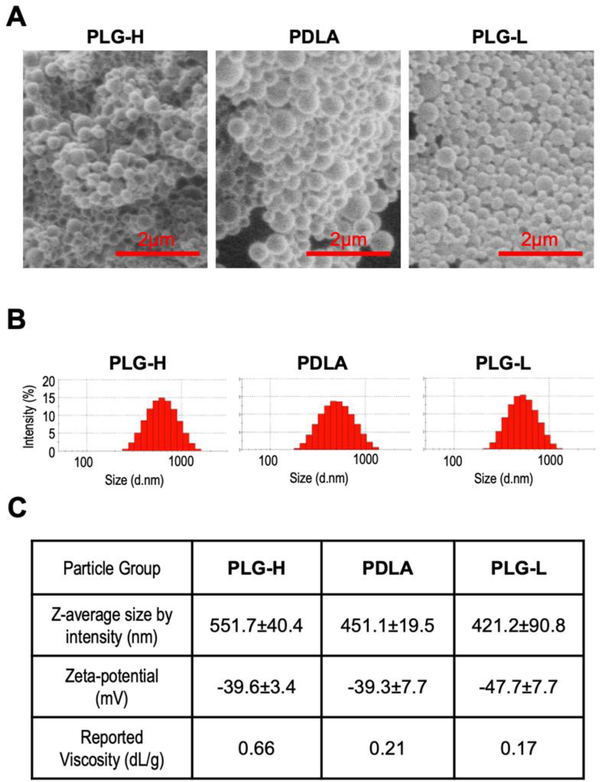
We created three types of nanoparticles, referred as PLG-H, PLG-L, and PDLA based on their various compositions of lactide and glycolide, and their inherent viscosity, a property associated with the polymer’s molecular weight. PLG was composed of a 50:50 ratio of glycolide to lactide, and PLG-H had a higher viscosity than PLG-L. PDLA had similar viscosity to PLG-L, yet was only composed of lactide. All particles were fabricated using a single emulsion process, with the surfactant poly(ethylene-alt-maleic anhydride) (PEMA). PEMA was used as a surfactant in order to achieve high negative charge on the particle [34,37]. Scanning electron microscope (SEM) images show that spherical shapes of fabricated PLG-H, PDLA, and PLG-L particles (Fig. 1A). Furthermore, the dynamic light scattering (DLS) data of the fabricated particles indicated a similar average diameter, ranging from 400 to 500 nm, and zeta-potential of approximately −40 mV or less (Fig. 1B, C).
Fig. 1.
Characterization of fabricated particles. (A) SEM images of PLG-H (left), PDLA (middle), and PLG-L (right) particles show spherical shapes of the fabricated particles. Scale bar indicates 2μm. (B) Sizes of PLG-H (left), PDLA (middle), and PLG-L (right) particles were measured by DLS. (C) The average size, surface charges, and reported inherent viscosity were shown. Z-average size and Zeta-potential values were measured 3–4 batches of particle fabrication.
3.2. Particle materials determined their internalization by blood leukocytes in vitro
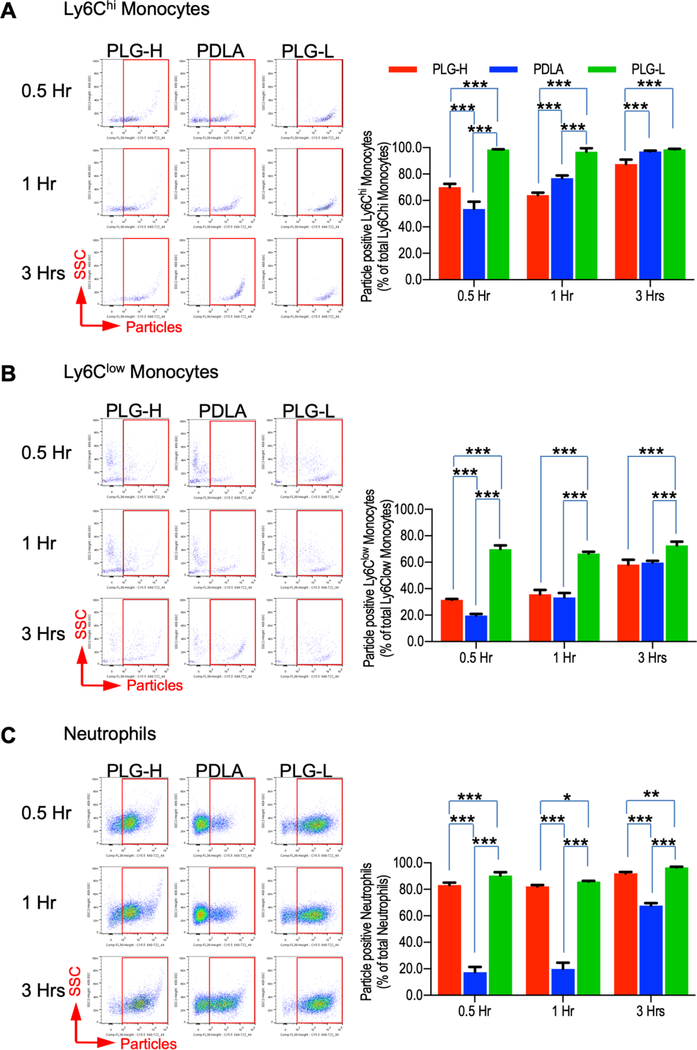
We initially investigated the response of blood leukocytes to particles in vitro, with the leukocytes obtained from mice at the initial stages of EAE where the numbers of inflammatory monocytes and neutrophils increase in the circulation, and are maximal prior to the onset of EAE [38,39]. We tested two in vitro particles concentrations (20 and 200 μg/mL), as particle concentration influenced the internalization and activity of both monocyte-derived macrophages and neutrophils [40,41]. Blood leukocytes were isolated at day 9 following disease induction, which is one or two days prior to clinical disease onset, and then were incubated with particles for 0.5, 1, and 3 hours, either at 20 or 200 μg/mL. The percentage of Ly6Chi monocytes, Ly6Clow monocytes, and neutrophils initially associated with particles varied with respect to the particle composition (Fig. 2A-C). In the blood, Ly6Chi monocytes and neutrophils are known as major immune cells which migrate into the inflamed tissue, while the function of Ly6Clow monocytes are patrolling on the endothelial layers and secreting cytokines and chemokines to recruit neutrophils upon inflammation [2,3]. Overall, PLG-L particles had greater association with Ly6Chi monocytes compared with PLG-H and PDLA particles (Fig. 1A). Interestingly, the percentage of Ly6Clow monocytes had significantly reduced association to PDLA and PLG-H particles compared with PLG-L particles at a dose of 20μg/mL (Fig. 2B), whereas neutrophils had a reduced association primarily for PDLA (Fig. 2C). At a dose of 200μg/mL, nearly all Ly6Chi monocytes and neutrophils were associated with all particles types at the initial time point and subsequent times (Fig. S1A, C), while association of Ly6Clow monocytes with PDLA particles were lower than PLG-H and PLG-L particles (Fig. S1B). Collectively, these in vitro data indicate preferential association of particles with specific cell types as a function of the polymer composition.
Fig. 2.
Particle association with monocytes and neutrophils in vitro. Blood cells were collected from EAE mice at 9 days p.i. and following red blood cell lysis, 20ug/mL of PLG-H PDLA, and PLG-L particles were added and incubated for 0.5, 1 and 3 hours. Representative flow cytometry plots (left) and percent (right) of (A) Ly6Chi monocytes (CD45+/CD11b+/Ly6G−/Ly6Chi), (B) Ly6Clow monocytes (CD45+/CD11b+/Ly6G−/Ly6Clow), and (C) neutrophils (CD45+/CD11b+/ Ly6G+) which were associated with particles. Particle positive cells were identified via gating cy5.5 positive cells. All data represent mean ± standard deviation (SD) (N = 4 per group).
3.3. Daily infusion of PLG-H particles to modulate EAE disease course
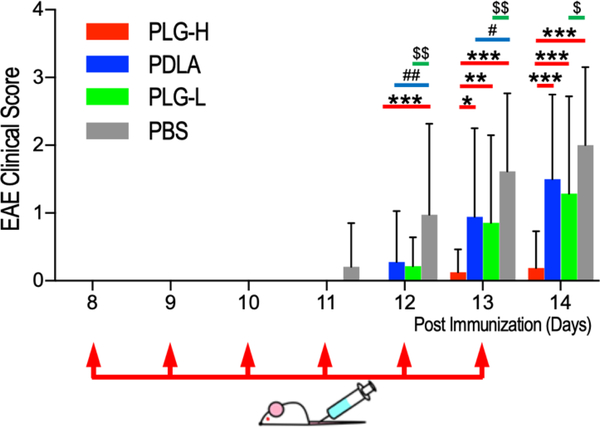
The impact of particles on the amelioration of inflammatory responses was subsequently investigated using the murine EAE model. The disease was initiated by immunization with the myelin peptides, proteolipid protein (PLP139–151), which ultimately leads to inflammatory demyelination in the CNS and neurologic dysfunction, similar to MS [42]. SJL/J mice were immunized with PLP131–159, and then particle injections were initiated at day 8 post EAE immunization (p.i.) prior to the onset of clinical signs, and continued until day 13 p.i., a time frame in which an increased frequency of neutrophils and monocytes precedes the onset of clinical disease [32]. No animal deaths or tissue necrosis due to particle injections were observed in this study. Signs of disease were observed from day 11 through day 14 p.i. among EAE mice treated with PBS (Fig. 3 and Fig. S2). Interestingly, PLG-H particle treatment led to significantly lower EAE scores than PBS control from day 12 through day 14 p.i, and PLG-L and PDLA particles on days 13 and 14 p.i. These data suggested that PLG-H particles were able to significantly reduce this initial stage of EAE compared with PLG-L and PDLA particles and PBS control (Fig. 3, red bar). PLG-L particles had lower clinical scores than PBS from 12, through 14 days p.i. (Fig. 3, green bar), while PDLA particles were significantly different relative to PBS on 12 and 13 days p.i. (Fig. 3, blue bar), suggesting that PDLA particles had the least effect on decreasing disease onset. Subsequent experiments focused on more detailed mechanisms of action employed PLG-H and PDLA particles as these two compositions had the greatest and smallest effect respectively in the EAE clinical scores.
Fig. 3.
Clinical scores of EAE mice receiving 6 consecutive days of particle infusion. EAE mice were intravenously injected with particles (PLG-L, PLG-H and PDLA) or PBS control starting day 8 p.i. until day 13 p.i. as indicated by the red arrows. PLG-H particles were compared with PLG-L particles, PDLA particles and PBS between 8 and 14 days p.i. (red bar with *). PDLA is compared with PLG-L and PBS (blue bar with p values of # < 0.05, ## < 0.01). PLG-L particles compared with PBS (green bar with p values of $ < 0.05, $ $ < 0.01). (N= 14 −22 per group). All data represent mean ± standard deviation (SD).
3.4. Particle internalization by blood leukocytes in vivo
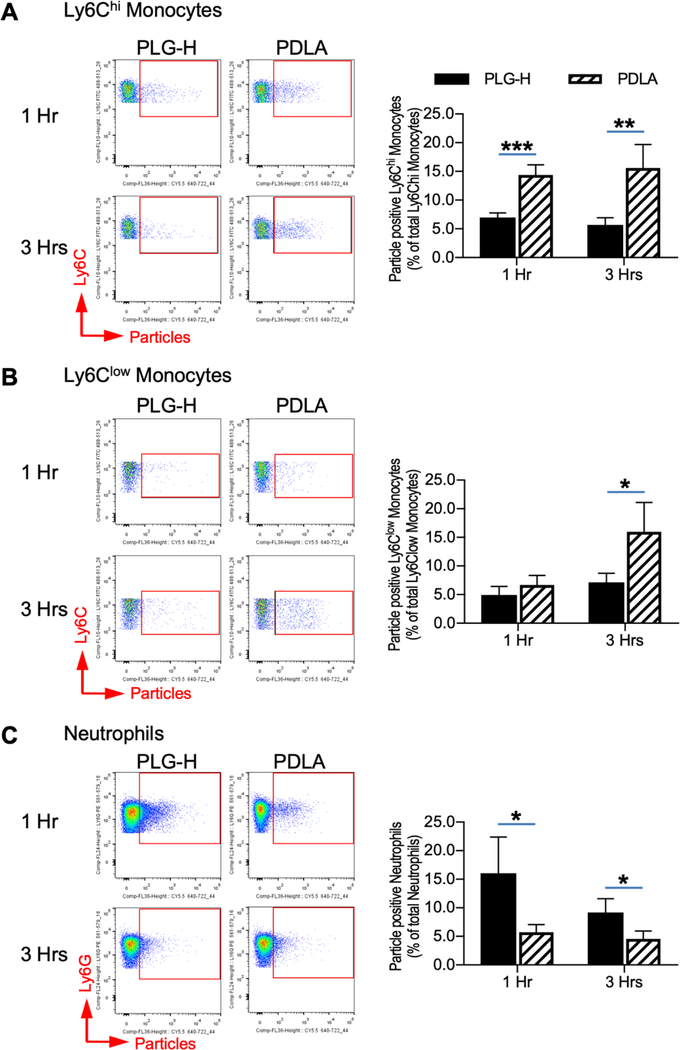
We subsequently investigated internalization of particles by monocytes and neutrophils in the blood, which have been reported to increase prior to EAE onset [32,38], following treatment with particles. The populations of Ly6Chi monocytes, Ly6Clow monocytes, and neutrophils as a percent of total immune cells in the blood were not different between PLG-H and PDLA particle treatment at any time point (Fig. S3). PDLA particles preferentially associated with Ly6Chi and Ly6Clow monocytes relative to PLG-H particles (Fig. 4A, B). The increased association of PLG-H particles with neutrophils was significant at 1 and 3 hour time points (Fig. 4C). In vivo particles association with Ly6Chi monocytes and neutrophils at the 1 and 3 hour time points agreed with the in vitro data (Fig. 2A, C). Analysis of CD45+ cells revealed that approximately 2–3% of CD45+ cells in the blood were particle positive, and no significant differences were present between groups (Fig. S4A). Within this PLG-H particle positive CD45+ cells, approximately 70–80% were neutrophils, while the percentage of PDLA positive neutrophils was approximately 50% (Fig. S4B). In addition to monocytes and neutrophils, other cells such as B-cells and eosinophils may associate with particles. PLG-H particles were phagocytosed by neutrophils to a greater extent compared with PDLA particles supporting PLG-H particles are preferably phagocytosed by neutrophils. PDLA particles showed greater association with Ly6Chi and Ly6Clow monocytes, but not with neutrophils at 24 hours after injection (Fig. S5A, B). This may be due to the mean circulation time for neutrophils being approximately 12.5 hours in mouse [4], and neutrophils associated with particles may be cleared from circulation as seen in reduction of neutrophil population at 24 hours.
Fig. 4.
Blood inflammatory and residential monocytes and neutrophils associated with particles in vivo. PLG-H and PDLA particles were injected to EAE mice at 10 days p.i., and then blood was collected at 1 and 3 hours after the injections. Representative flow cytometry plots (left) and percent (right) of (A) Ly6Chi monocytes (CD45+/CD11b+/Ly6G−/Ly6Chi), (B) Ly6Clow monocytes (CD45+/CD11b+/ Ly6G−/Ly6Clow), and (C) neutrophils (CD45+/CD11b+/Ly6G+) which were associated with particles. Particle positive cells were identified via gating cy5.5 positive cells. All data represent mean ± standard deviation (SD) (N = 4 per group).
3.5. PLG-H particles reduced immune cells in the CNS
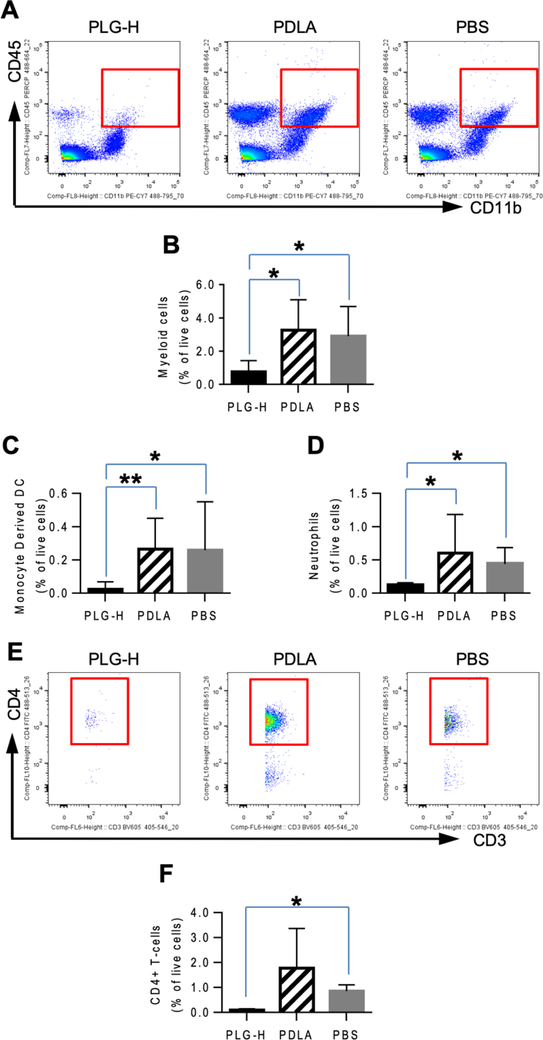
We next investigated whether the amelioration of EAE disease coincided with the reduction in the numbers of leukocytes and myeloid cells in the CNS [23]. At day 14 p.i., which corresponds to one day after termination of daily particle infusions, treatment with PLG-H particles resulted in lower number of CD45+/CD11b+ myeloid cells in the CNS than with treatment by PDLA particles or the PBS control (Fig. 5A, B), consistent with the EAE clinical scores (Fig. 3). Furthermore, the population of inflammatory Ly6Chi monocyte-derived dendritic cells (DCs) in the CNS, which typically function to induce differentiation of CD4+ T-cells toward Th1 and Th17 [43], was significantly lower in the CNS of mice treated with PLG-H particles than with the other groups (Fig. 5C). In addition, the population of neutrophils in the CNS was significantly lower following treatment with PLG-H particles compared to PDLA particles and PBS control (Fig. 5D).
Fig. 5.
Myeloid cells and lymphocytes in CNS at day 14 p.i., which was 24 hours after 6 consecutive days of particle infusions. (A) Representative flow cytometry plots show that population of myeloid cells (CD45+/CD11b+) among live cells. Percent of (B) myeloid cells, (C) monocyte-derived dendritic cells (CD45+/CD11b+/Ly6C+/CD11c+) and (D) neutrophils (CD45+/CD11b+/Ly6G+) among live cells are shown. (E) Representative flow cytometry plots show the population of CD4+ T-Cells (CD45+/CD11b−/CD3+/CD4+) among live cells. (F) Percentage of CD4+ T-cell among live cells. All data represent mean ± standard deviation (SD) (N = 7–8 per group).
Myelin damage is mediated by CD4+ T-cells [35,44], and we thus investigated their presence within the CNS. PLG-H particle treatment led to significantly lower populations of CD4+ T-cells than PBS control, while PDLA particle treatment showed no reduction compared to PBS control (Fig. 5E, F) supporting treatment with PLG-H particles decreased migration of monocytes derived DC and neutrophils led to reduction of CD4+ T-cells to ameliorate the disease. This result suggests that the reduced clinical signs observed may be due in part to an overall reduction in CD4+ T-cell numbers in the CNS. Collectively, the daily injection of PLG-H particles resulted in significant reduction in the population of inflammatory monocytes and neutrophils, and a concomitant decrease in the number of CD4+ T-cells in the CNS.
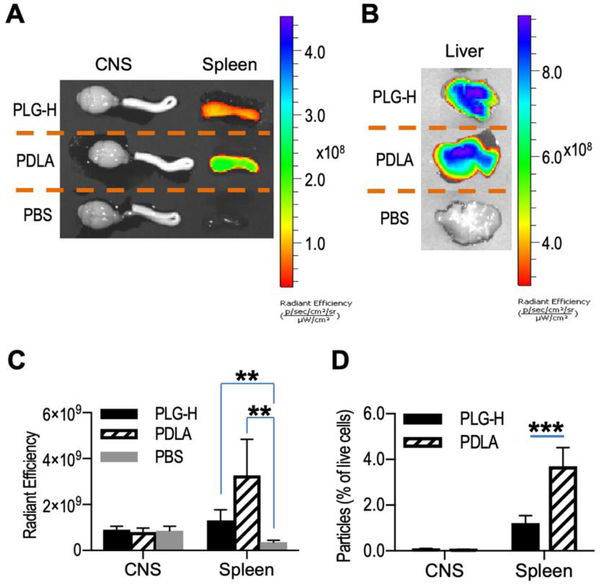
3.6. Particle biodistribution and association with myeloid cells in the CNS and spleen
Reduced migration of inflammatory cells to the CNS suggested their accumulation elsewhere, which motivated studies to identify the biodistribution of the particles. The particles were labeled with cyanine 5.5 dye, infused over 6 days as previously noted. A greater fluorescence intensity was observed in the spleen and liver following treatment with fluorescently-labeled particles compared to PBS (Fig. 6A, B). The CNS did not have substantial fluorescence signal at 14 day p.i. which was one day after the end of particle infusion (Fig. 6A), agreeing with reports that particles with a diameter of approximately 500 nm are cleared by the reticuloendothelial systems and accumulate in the liver and spleen [45,46]. The fluorescence intensity in the spleen was greater following injection of PDLA particles compared to injection of PLG-H particles (Fig. 6C). The higher fluorescent intensity may be because PDLA particles persist longer in the spleen and liver than PLG-H particles due to slower degradation [47,48]. In addition, fluorescence-activated cell sorting (FACS) analysis indicated that more PDLA particles than PLG-H particles were associated with cells in the spleen agreeing with the IVIS data (Fig. 6D). Although a greater population of myeloid cells, monocyte-derived DCs and neutrophils was present in the CNS following treatment with PDLA particles relative to PLG-H (Fig. 5C, D), the number of particle positive cells was negligible similar to PLG-H particles (Fig. 6C, D) suggesting that neutrophils and inflammatory monocytes, which do not phagocytose particles, migrate and cause inflammation in the CNS.
Fig. 6.
Particle biodistribution in CNS, spleen and liver. Particle biodistribution in CNS, spleen (A) and liver (B) were evaluated at 24 hours after particle infusion (PLG-H and PDLA particles) or PBS control using a IVIS Lumina LET camera system. Blue color indicates greater accumulation of particles. (C) Radiant efficiency of PLG-H particles, PDLA particles and PBS control in both CNS and spleen were calculated. (D) Population of live cells associated with particles in CNS and spleen were determined using flow cytometry. All data represent mean ± standard deviation (SD) (N = 7–8 per group).
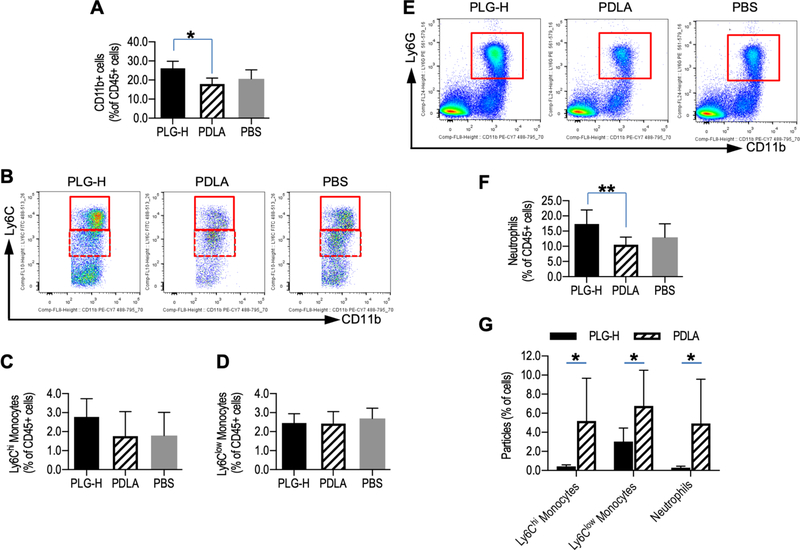
3.7. PLG-H particles redirected more neutrophils to the spleen
For the spleen, the accumulation of particles coincided with a significant increase in the number of myeloid cells (CD45+/CD11b+) following treatment with PLG-H particles compared to PDLA particles (Fig. 7A). Treatment with PLG-H particles result in a trend toward a greater population of Ly6Chi monocytes (CD45+/CD11b+/Ly6G−/Ly6Chi) than either PDLA particles or PBS (Fig. 7B, C), while the population of Ly6Clow monocytes (CD45+/CD11b+/Ly6G−/Ly6Clow) were similar between the groups (Fig. 7D). The population of neutrophils (CD45+/CD11b+/Ly6G+) in the spleen was significantly greater for PLG-H particle treatment relative to PDLA particle treatment, and trended toward greater number than the PBS control, yet was not significant (p = 0.104) (Fig. 7E, F). The data indicates that more neutrophils were associated with PLG-H particles and redirected to the spleen. In contrast, the percentage of CD4+ T-cells in the splenic population was not significantly different for PLG-H particles relative to PDLA particles (Fig. S6A, B) indicating that trafficking of CD4+ T-cells was not redirected to the spleen by particle treatment. Within the spleen, both PLG-H and PDLA particles were detected as association with Ly6Chi monocytes, Ly6Clow monocytes and neutrophils (Fig. 7G). Interestingly, the percentages of Ly6Chi monocytes and Ly6Clow monocytes association with PDLA particles were greater than those with PLG-H particles. Since there is not significant difference of the monocyte population between PLG-H and PDLA particles, slower degradation of PDLA may associate with Ly6Chi monocytes and Ly6Clow monocytes in spleen and did not contribute redirecting the monocytes.
Fig. 7.
Monocytes and neutrophils in spleen at day 14 p.i., which was 24 hours after 6 consecutive days of particle infusions. (A) Population of myeloid cells (CD45+/CD11b+) among live cells in the spleen were shown. (B) Representative flow cytometry plots showing that population of Ly6Chi monocytes (CD45+/CD11b+/Ly6G−/Ly6Chi) (solid box) and Ly6Clow monocytes (CD45+/CD11b+/Ly6G−/Ly6Clow) (dotted box), and population of (C) Ly6Chi monocytes and (D) Ly6Clow monocytes among live cells were shown. (E) Representative flow cytometry plots of neutrophils (CD45+/CD11b+/Ly6G+) and (F) greater neutrophil populations were in PLG-H particle group are shown. (G) Percent of Ly6Chi monocytes, Ly6Clow monocytes and neutrophils associated with PLG-H or PDLA particles were calculated. All data represent mean ± standard deviation (SD) (N = 7–8 per group).
4. Discussion
Acute inflammation is central to a number of immune disorders and traumatic injuries, and modulation of this inflammatory response offers therapeutic options. Strategies are emerging that target innate inflammatory cells in the blood, specifically, inflammatory monocytes and neutrophils, with the intention of reducing trafficking to sites of cell damage and inflammation. Interestingly, a recent study reported that PLG particles, carrying no active pharmaceutical agent, delivered intravenously, were able to ameliorate pathogenic inflammation from EAE, inflammatory bowel disease, cardiac or kidney reperfusion, spinal cord injury or viral infection [23,24,49]. Inflammatory cells, such as monocytes and neutrophils, can phagocytose particles in the blood to remove them from circulation. While these studies demonstrate the potential of one type of commercially available PLG particles without an active drug to attenuate acute inflammation, identifying the PLG-based particle design parameters and mechanisms of action can facilitate the application of particles to modulate inflammation. The composition of the particle can influence cell responses such as internalization and the subsequent cell phenotype [50–52], and we thus investigated the polymer composition for its impact on modulation of inflammation related with leukocyte association and phenotypes. Our study uniquely demonstrated that not all PLG-based particles were able to modulate inflammation and suggests that higher molecular weight of PLG-based particles is required for modulation of inflammation.
In the present study, nanoparticles varying in monomer ratio and molecular weight, but similar in size and charge, produced distinctly different outcomes in immune cells and inflammatory responses. The internalization of nanoparticles is influenced by their physicochemical properties [53]. Numerous types of nanoparticles (e.g., carbon nanotubes, iron oxide and albumin) have been observed to be differentially internalized by monocytes and neutrophils [54–57], similar to what we have observed here. The composition and molecular weight of PLG, as well as the nanoparticle concentration, affected the particle association with inflammatory monocytes and neutrophils. We found that the effects of composition on internalization by myeloid lineage cells were similar between in vitro and in vivo experiments, where PLG-H particles were associated with neutrophils to a greater extent than PDLA particles (Fig. 2C). In contrast, PDLA particles had greater association with inflammatory monocytes (Fig. 2A). These findings were agreeing with the previous reports that the properties of PLG particles, including degradation and hydrophobicity, depends on the ratio of lactide and glycolide as well as the molecular weight, which can then determine the interaction that these particles have with immune cells [50,58,59]. Then, in combination with the data showing that PLG-H particles ameliorate EAE disease better than PDLA particles, our study suggested that more hydrophilic particles (PLG-H particles) are preferentially internalized by neutrophils which largely function to ameliorate inflammation. We have also seen that PLG-L were internalized by both inflammatory monocytes and neutrophils compared to PLG-H and PDLA particles. However, PLG-L particles did not significantly ameliorate EAE disease compared with PLG-H particles despite similar internalization by neutrophils in vitro, suggesting that the impact is a combination of internalization and phenotypic polarization. Collectively, PLG-H particles in the present study functioned similarly at modulating inflammation to the commercially available PLG particles and other non-degradable particles reported in our previous study [23].
The differential responses observed with the particles likely represents some combination of internalization by distinct routes or differential activation of signaling pathways that influences polarization. Previous studies have demonstrated particle internalization through distinct receptors such as the MARCO receptor, which is non-opsonic phagocytic receptor and can modulate immune cell responses [23,28]. While, it is worth noting that pathways for internalization of nanoparticles include, but are not limited to, clathrin- and caveolae-mediated endocytosis, phagocytosis and micropinocytosis [60]. Particle properties such as hydrophobicity can influence the proteins that adsorb to the particle, which can then influence the specific pathway through which the particles are internalization. The various particle internalization ratios by inflammatory monocytes and neutrophils may result from the variations in hydrophobicity that influence this composition of the corona. For example, apolipoprotein is one of the most common proteins that associate with nanoparticles, and the levels of the protein can influence clearance of particles by immune cells [61,62]. Following internalization, particles may also activate distinct cellular pathways such as endosome and lysosome [63]. In macrophages, most particles turn out to be in the lysosome, in which composition and molecular weight of PLGA-based materials varies degradation and acidity of particles resulting in affect lysosomal pH [64]. Furthermore, degradation leads to release of lactic acid, which others have reported as associated with a delay in LPS-induced genes, reduced NF-κB nuclear localization, and modified effector proteins such as TNF-α and IL-23 or chemokines such as MCP-1 and CCL7 [65]. A previous reported indicated that PLG particles cultured with LPS treated macrophages had levels of Caspase-1 similar to control, suggesting a deactivation of the inflammasome [66].
The nanoparticles associated with monocytes in the blood and subsequently reduced myeloid cells and monocyte-derived dendritic cells in the CNS, which correlated with the reduction of EAE clinical score. Monocytes are recruited to the CNS in EAE to induce disease as a result of their migration through the BBB and infiltration into the brain [67], and the intensity of this infiltrate is associated with EAE severity [38,39]. Consistent with this mechanism of disease initiation, depletion of circulating inflammatory monocytes during the effector phase of EAE has been shown to improve the clinical score in the disease [38,68]. Previous studies proposed that monocytes and neutrophils phagocytose particles in blood circulation, undergo apoptotic change and are, as a result, sequestered in the spleen or liver for elimination, thereby delaying the infiltration of these cells into the CNS to drive inflammation [23,27]. While our results agree overall with the previous reports [23], we observed that PLG-H particles were more effective than the PLG-L and PDLA particles in reducing clinical disease scores. PLG-H particle treatment clearly reduced innate immune cell infiltration into the CNS, while the numbers of these cells were not significantly diminished by PDLA particles despite the PDLA particles inducing a greater accumulation in the spleen by the end of the treatment course. This greater association may reflect in part the slower degradation of the PLA polymer, which would facilitate quantification due to an increase of its persistence in cells [47,48]. Importantly, although a greater population of myeloid cells, monocyte-derived DCs and neutrophils in the CNS treated with PDLA particles, the number of particle positive cells were negligible, similar to PLG-H particles, suggesting that the majority of such cells were sequestered in the spleen or liver (Fig. 6). The population of myeloid cells in the spleen was not greater for particle treated animals compared to the PBS control (Fig. 7). Differences in myeloid number would be difficult to discern given the substantially larger number of myeloid cells in the spleen relative to the other organs, though it is possible that inflammatory monocytes undergo apoptosis in the spleen. An alternative hypothesis is that the particle positive cells are impacting the environment in the spleen and the subsequent mobilization of local myeloid cells [69]. Overall, these findings also suggest that neutrophils and inflammatory monocytes, which do not phagocytose particles, migrate and cause inflammation in the CNS. PDLA particles were associated with inflammatory monocytes to a greater extent than PLG-H in vivo (Fig. 4) agreeing with in vitro data (Fig. 2), yet PDLA particles did not reduce EAE scores to the same extent.
Short term in vivo experiments indicated a greater proportion of inflammatory monocytes in the blood internalized PDLA particles, while more neutrophils internalized PLG-H particles. Greater internalization of PLG-H particles by neutrophils in a short incubation time is supported by a previous study reporting about 80% of neutrophils internalize PLG particles in 15 minutes of incubation time [70]. Neutrophils infiltrate the CNS a few days before EAE disease onset, triggering circulation of inflammatory monocyte which promotes to macrophages or DCs, and depletion of neutrophils can attenuate the severity of EAE [5,71]. Since the population of neutrophils infiltrating the CNS were significantly decreased in PLG-H particle treatment groups (Fig. 5), we speculate that PLG-H particle treatment is more effective due to a two-stage mechanism of action, in which neutrophils are initially reduced, substantially diminishing the initial inflammatory insult which would normally be amplified by the subsequent infiltration of inflammatory monocytes to produce full-brown EAE. This proposed mechanism is supported by the greater efficacy of the PLG-H particles, compared to PDLA treatment where modest reduction of clinical signs is observed only later in disease onset. The reduction of neutrophil infiltration and amelioration of EAE observed with the PLG-H particles is consistent with a previous study using particles to reduce disease severity in acute lung injury models [27]. Although neutrophils are not antigen presenting cells, they indirectly activate T-cells via secreting cytokines, such as TNF-α, IL-6 and IFN-γ, which promote maturation of antigen presenting cells (APCs) in the CNS. The matured APCs activate CNS-infiltrating disease specific T-cells via presentation of myelin antigen [71,72]. In addition, neutrophils contribute to breakdown of the BBB at the acute stage of EAE disease [73]. These facts suggest that while particle uptake by inflammatory monocytes may be necessary for disease amelioration, it may not be sufficient to retard infiltration of these cells into the brain without the early reduction in neutrophils. Finally, although cell specificity is typically achieved through surface modifications and conjugation of antibodies on the particles [70], our data demonstrate cell selectivity that depends on particle composition. PDLA particles had less association with neutrophils than PLG-H particles at 1 and 3 hours in vivo (Fig. 4) and their CNS neutrophils numbers significantly increased compared to PLG-H particles (Fig. 5), agreeing that neutrophil poorly internalize PLA particles without plasma protein opsonization of the particle surface [40]. Collectively, particles targeting neutrophils rather than inflammatory monocytes may be a necessary factor to ameliorate EAE disease. Our research targeting blood immune cells can be also applied to other neurodegenerative disease including stroke, Alzheimer’s and Parkinson’s diseases in which nanoparticles are targeting to pass through BBB for the treatment [74].
5. Conclusion
The present study investigated design parameters of drug-free particles to target and modulate inflammatory monocytes and neutrophils to reduce the severity of inflammation in a murine EAE model of MS. Investigation of variations of molecular weight and composition of PLG based particles clearly showed that PLG-H particles maximally ameliorate EAE compared to PLG-L and PDLA particles. Furthermore, in vivo and in vitro data indicate that particle association with blood monocytes and/or neutrophils is related to particle composition. Inflammatory monocytes preferentially associated with the relatively more hydrophobic PDLA particles, while neutrophils associated preferentially with the relatively more hydrophilic PLG-H and PLG-L particles. The ability of PLG-H particles to reduce clinical scores more effectively than the PDLA particles relied on internalization by both neutrophils and inflammatory monocytes. Our results suggest that designing particles which efficiently modulate blood neutrophils and inflammatory monocytes is critical to reduce the initial inflammation by neutrophils and the generation of DC from inflammatory monocytes in the CNS. In turn, this reduce antigen presentation to activated CD4+ T-cells, thereby reducing T-cell proliferation, which results in maximal reduction in disease severity. Notably, these nanoparticles do not require an active pharmaceutical ingredient and intravenous infusion of these PLG based particles provides a potential avenue to treat acute inflammatory diseases.
Supplementary Material
Fig. S1. Particle association with monocytes and neutrophils in vitro. Blood cells were collected from EAE mice at 9 days p.i. and following red blood cell lysis, 200ug/mL of PLG-H PDLA, and PLG-L particles were added and incubated for 0.5, 1 and 3 hours. Representative flow cytometry plots (left) and percent (right) of (A) Ly6Chi monocytes (CD45+/CD11b+/Ly6G−/Ly6Chi), (B) Ly6Clow monocytes (CD45+/CD11b+/Ly6G−/Ly6Clow), and (C) neutrophils (CD45+/CD11b+/ Ly6G+) which were associated with particles were also calculated via gating cy5.5 positive cells. All data represent mean ± standard deviation (SD) (N = 4 per group).
Fig. S2. Clinical scores of EAE mice receiving consecutive 6 days of particle infusion. EAE mice were intravenously injected with particles (PLG-L, PLG-H and PDLA) or PBS control starting day 8 p.i. until day 13 p.i. The data plots clinical scores for PLG-H particles (red square), PDLA (blue triangle), PLG-L (green circle) and PBS (black cross).
Fig. S3. Particle association with monocytes and neutrophils in the blood. PLG-H and PDLA particles were injected to EAE mice at 10 days p.i., and then blood was collected at 1 and 3 hours after the injections. Representative flow cytometry plots of (A) Ly6Chi monocytes, and Ly6Clow monocytes, and population of (B) Ly6Chi monocytes and (C) Ly6Clow monocytes in CD45+ cells were determined. (D) Representative flow cytometry plots and population of (E) neutrophils in CD45+ cells were determined. All data represent mean ± standard deviation (SD) (N = 4 per group).
Fig. S4. (A) Population of particle positive CD45+ cells in total CD45+ cells in the blood. (B) Proportion of particle associated Ly6Chi monocytes, Ly6Clow and neutrophils in CD45+ cells in the blood.
Fig. S5. Particle association with monocytes and neutrophils in the blood. PLG-H and PDLA particles were injected to EAE mice at 24 hours after consecutive particle injections. (A) Population of Ly6Chi monocytes, Ly6Clow monocytes, and neutrophils in CD45+ cells were determined. (B) Representative flow cytometry plots (left) and population (right) of Ly6Chi monocytes, Ly6Clow monocytes, and neutrophils associated with particles were determined. All data represent mean ± standard deviation (SD) (N = 4 per group).
Fig. S6. CD4+ T-cells in the spleen. (A) Representative flow cytometry plots showing how subsets of CD4+ T-cells were gated. (B) Population of CD4+ T-cell among live cells were calculated. All data represent mean ± standard deviation (SD) (N = 4 per group).
Drug-free biodegradable particles modulate inflammation, though the design matters.
High molecular weight polymeric particles ameliorated EAE.
Particle positive cells were observed in the spleen and not the CNS for EAE.
Particles effective at attenuating EAE preferentially associated with neutrophils.
Acknowledgements
This work was supported by NIH grants R01EB013198 (to L.D.S and S.D.M). We wish to thank the Flow Cytometry Core at the University of Michigan, Biomedical Research Core Facilities.
Footnotes
Conflicts of Interest
L.D.S., S.D.M and N.J.C.K have financial interests in Cour Pharmaceutical Development Company.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference:
- [1].Serbina NV, Jia T, Hohl TM, Pamer EG, Monocyte-mediated defense against microbial pathogens, Annu.Rev.Immunol 26 (2008) 421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shi C, Pamer EG, Monocyte recruitment during infection and inflammation, Nat.Rev.Immunol 11 (2011) 762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Soehnlein O, Lindbom L, Phagocyte partnership during the onset and resolution of inflammation, Nat.Rev.Immunol 10 (2010) 427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- [4].Ginhoux F, Jung S, Monocytes and macrophages: developmental pathways and tissue homeostasis, Nat.Rev.Immunol 14 (2014) 392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- [5].Kolaczkowska E, Kubes P, Neutrophil recruitment and function in health and inflammation, Nat.Rev.Immunol 13 (2013) 159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- [6].Ingersoll MA, Platt AM, Potteaux S, Randolph GJ, Monocyte trafficking in acute and chronic inflammation, Trends Immunol. 32 (2011) 470–477. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D, Immunopathologic alterations in murine models of sepsis of increasing severity, Infect.Immun 67 (1999) 6603–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Marik PE, Early management of severe sepsis: concepts and controversies, Chest. 145 (2014) 1407–1418. doi: 10.1378/chest.13-2104. [DOI] [PubMed] [Google Scholar]
- [9].Fu Y, Liu Q, Anrather J, Shi FD, Immune interventions in stroke, Nat.Rev.Neurol 11 (2015) 524–535. doi: 10.1038/nrneurol.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mc Carthy DJ, Malhotra M, O’Mahony AM, Cryan JF, O’Driscoll CM, Nanoparticles and the blood-brain barrier: advancing from in-vitro models towards therapeutic significance, Pharm.Res 32 (2015) 1161–1185. doi: 10.1007/s11095-014-1545-6. [DOI] [PubMed] [Google Scholar]
- [11].Kleiman A, Tuckermann JP, Glucocorticoid receptor action in beneficial and side effects of steroid therapy: lessons from conditional knockout mice, Mol.Cell.Endocrinol 275 (2007) 98–108. [DOI] [PubMed] [Google Scholar]
- [12].Tabansky I, Messina MD, Bangeranye C, Goldstein J, Blitz-Shabbir KM, Machado S, Jeganathan V, Wright P, Najjar S, Cao Y, Sands W, Keskin DB, Stern JN, Advancing drug delivery systems for the treatment of multiple sclerosis, Immunol.Res 63 (2015) 58–69. doi: 10.1007/s12026-015-8719-0. [DOI] [PubMed] [Google Scholar]
- [13].Luster AD, Alon R, von Andrian UH, Immune cell migration in inflammation: present and future therapeutic targets, Nat.Immunol 6 (2005) 1182–1190. [DOI] [PubMed] [Google Scholar]
- [14].Hubbell JA, Thomas SN, Swartz MA, Materials engineering for immunomodulation, Nature. 462 (2009) 449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- [15].Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, Getts MT, Pleiss M, Luo X, King NJ, Shea LD, Miller SD, Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis, Nat.Biotechnol 30 (2012) 1217–1224. doi: 10.1038/nbt.2434; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ, Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis, Proc.Natl.Acad.Sci.U.S.A 109 (2012) 11270–11275. doi: 10.1073/pnas.1120611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yuan B, Zhao L, Fu F, Liu Y, Lin C, Wu X, Shen H, Yang Z, A novel nanoparticle containing MOG peptide with BTLA induces T cell tolerance and prevents multiple sclerosis, Molecular Immunology. 57 (2014) 93–99. doi: 10.1016/j.molimm.2013.08.006. [DOI] [PubMed] [Google Scholar]
- [18].Langert KA, Goshu B, Stubbs EB Jr, Attenuation of experimental autoimmune neuritis with locally administered lovastatin-encapsulating poly(lactic-co-glycolic) acid nanoparticles, J.Neurochem 140 (2017) 334–346. doi: 10.1111/jnc.13892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nakano Y, Matoba T, Tokutome M, Funamoto D, Katsuki S, Ikeda G, Nagaoka K, Ishikita A, Nakano K, Koga J, Sunagawa K, Egashira K, Nanoparticle-Mediated Delivery of Irbesartan Induces Cardioprotection from Myocardial Ischemia-Reperfusion Injury by Antagonizing Monocyte-Mediated Inflammation, Sci.Rep 6 (2016) 29601. doi: 10.1038/srep29601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kelly C, Yadav AB, Lawlor C, Nolan K, O’Dwyer J, Greene CM, McElvaney NG, Sivadas N, Ramsey JM, Cryan SA, Therapeutic aerosol bioengineering of siRNA for the treatment of inflammatory lung disease by TNFalpha gene silencing in macrophages, Mol.Pharm 11 (2014) 4270–4279. doi: 10.1021/mp500473d. [DOI] [PubMed] [Google Scholar]
- [21].Howard MD, Hood ED, Zern B, Shuvaev VV, Grosser T, Muzykantov VR, Nanocarriers for vascular delivery of anti-inflammatory agents, Annu.Rev.Pharmacol.Toxicol 54 (2014) 205–226. doi: 10.1146/annurev-pharmtox-011613-140002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wischke C, Schwendeman SP, Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles, Int.J.Pharm 364 (2008) 298–327. doi: 10.1016/j.ijpharm.2008.04.042. [DOI] [PubMed] [Google Scholar]
- [23].Getts DR, Terry RL, Getts MT, Deffrasnes C, Muller M, van Vreden C, Ashhurst TM, Chami B, McCarthy D, Wu H, Ma J, Martin A, Shae LD, Witting P, Kansas GS, Kuhn J, Hafezi W, Campbell IL, Reilly D, Say J, Brown L, White MY, Cordwell SJ, Chadban SJ, Thorp EB, Bao S, Miller SD, King NJ, Therapeutic inflammatory monocyte modulation using immune-modifying microparticles, Sci.Transl.Med 6 (2014) 219ra7. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jeong SJ, Cooper JG, Ifergan I, McGuire TL, Xu D, Hunter Z, Sharma S, McCarthy D, Miller SD, Kessler JA, Intravenous immune-modifying nanoparticles as a therapy for spinal cord injury in mice, Neurobiol.Dis 108 (2017) 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Webber MJ, Matson JB, Tamboli VK, Stupp SI, Controlled release of dexamethasone from peptide nanofiber gels to modulate inflammatory response, Biomaterials. 33 (2012) 6823–6832. doi: 10.1016/j.biomaterials.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Somasuntharam I, Boopathy AV, Khan RS, Martinez MD, Brown ME, Murthy N, Davis ME, Delivery of Nox2-NADPH oxidase siRNA with polyketal nanoparticles for improving cardiac function following myocardial infarction, Biomaterials. 34 (2013) 7790–7798. doi: 10.1016/j.biomaterials.2013.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fromen CA, Kelley WJ, Fish MB, Adili R, Noble J, Hoenerhoff MJ, Holinstat M, Eniola-Adefeso O, Neutrophil-Particle Interactions in Blood Circulation Drive Particle Clearance and Alter Neutrophil Responses in Acute Inflammation, ACS Nano. (2017). doi: 10.1021/acsnano.7b03190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kanno S, Furuyama A, Hirano S, A murine scavenger receptor MARCO recognizes polystyrene nanoparticles, Toxicol.Sci 97 (2007) 398–406. [DOI] [PubMed] [Google Scholar]
- [29].Ulery BD, Phanse Y, Sinha A, Wannemuehler MJ, Narasimhan B, Bellaire BH, Polymer chemistry influences monocytic uptake of polyanhydride nanospheres, Pharm.Res 26 (2009) 683–690. doi: 10.1007/s11095-008-9760-7. [DOI] [PubMed] [Google Scholar]
- [30].Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V, PLGA-based nanoparticles: an overview of biomedical applications, J.Control.Release 161 (2012) 505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- [31].Peres C, Matos AI, Conniot J, Sainz V, Zupancic E, Silva JM, Graca L, Sa Gaspar R, Preat V, Florindo HF, Poly(lactic acid)-based particulate systems are promising tools for immune modulation, Acta Biomater. 48 (2017) 41–57. [DOI] [PubMed] [Google Scholar]
- [32].Rumble JM, Huber AK, Krishnamoorthy G, Srinivasan A, Giles DA, Zhang X, Wang L, Segal BM, Neutrophil-related factors as biomarkers in EAE and MS, J.Exp.Med 212 (2015) 23–35. doi: 10.1084/jem.20141015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McCarthy DP, Yap JW, Harp CT, Song WK, Chen J, Pearson RM, Miller SD, Shea LD, An antigen-encapsulating nanoparticle platform for TH1/17 immune tolerance therapy, Nanomedicine. 13 (2017) 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lo CT, Van Tassel PR, Saltzman WM, Simultaneous release of multiple molecules from poly(lactide-co-glycolide) nanoparticles assembled onto medical devices, Biomaterials. 30 (2009) 4889–4897. doi: 10.1016/j.biomaterials.2009.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Miller SD, Karpus WJ, Davidson TS, Experimental autoimmune encephalomyelitis in the mouse, Curr.Protoc.Immunol Chapter 15 (2010) Unit 15.1. doi: 10.1002/0471142735.im1501s88. [DOI] [PubMed] [Google Scholar]
- [36].Smith CE, Miller SD, Multi-peptide coupled-cell tolerance ameliorates ongoing relapsing EAE associated with multiple pathogenic autoreactivities, J.Autoimmun. 27 (2006) 218–231. doi: 10.1016/j.jaut.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hunter Z, McCarthy DP, Yap WT, Harp CT, Getts DR, Shea LD, Miller SD, A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease, ACS Nano. 8 (2014) 2148–2160. doi: 10.1021/nn405033r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].King IL, Dickendesher TL, Segal BM, Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease, Blood. 113 (2009) 3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ, CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis, J.Immunol 179 (2007) 5228–5237. [DOI] [PubMed] [Google Scholar]
- [40].Jackson JK, Springate CM, Hunter WL, Burt HM, Neutrophil activation by plasma opsonized polymeric microspheres: inhibitory effect of pluronic F127, Biomaterials. 21 (2000) 1483–1491. [DOI] [PubMed] [Google Scholar]
- [41].Guedj AS, Kell AJ, Barnes M, Stals S, Goncalves D, Girard D, Lavigne C, Preparation, characterization, and safety evaluation of poly(lactide-co-glycolide) nanoparticles for protein delivery into macrophages, Int.J.Nanomedicine 10 (2015) 5965–5979. doi: 10.2147/IJN.S82205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tavazzi E, Rovaris M, La Mantia L, Drug therapy for multiple sclerosis, CMAJ. 186 (2014) 833–840. doi: 10.1503/cmaj.130727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chow KV, Lew AM, Sutherland RM, Zhan Y, Monocyte-Derived Dendritic Cells Promote Th Polarization, whereas Conventional Dendritic Cells Promote Th Proliferation, J.Immunol 196 (2016) 624–636. doi: 10.4049/jimmunol.1501202. [DOI] [PubMed] [Google Scholar]
- [44].Rao P, Segal BM, Experimental autoimmune encephalomyelitis, Methods Mol.Biol 900 (2012) 363–380. doi: 10.1007/978-1-60761-720-4_18. [DOI] [PubMed] [Google Scholar]
- [45].Ernsting MJ, Murakami M, Roy A, Li SD, Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles, J.Control.Release 172 (2013) 782–794. doi: 10.1016/j.jconrel.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Simon LC, Sabliov CM, The effect of nanoparticle properties, detection method, delivery route and animal model on poly(lactic-co-glycolic) acid nanoparticles biodistribution in mice and rats, Drug Metab.Rev 46 (2014) 128–141. doi: 10.3109/03602532.2013.864664. [DOI] [PubMed] [Google Scholar]
- [47].Shive MS, Anderson JM, Biodegradation and biocompatibility of PLA and PLGA microspheres, Adv.Drug Deliv.Rev 28 (1997) 5–24. [DOI] [PubMed] [Google Scholar]
- [48].Blanco MD, Sastre RL, Teijon C, Olmo R, Teijon JM, Degradation behaviour of microspheres prepared by spray-drying poly(D,L-lactide) and poly(D,L-lactide-co-glycolide) polymers, Int.J.Pharm 326 (2006) 139–147. [DOI] [PubMed] [Google Scholar]
- [49].Edwards RG, Kopp SJ, Ifergan I, Shui JW, Kronenberg M, Miller SD, Longnecker R, Murine Corneal Inflammation and Nerve Damage After Infection With HSV-1 Are Promoted by HVEM and Ameliorated by Immune-Modifying Nanoparticle Therapy, Invest.Ophthalmol.Vis.Sci 58 (2017) 282–291. doi: 10.1167/iovs.16-20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Walter E, Dreher D, Kok M, Thiele L, Kiama SG, Gehr P, Merkle HP, Hydrophilic poly(DL-lactide-co-glycolide) microspheres for the delivery of DNA to human-derived macrophages and dendritic cells, J.Control.Release 76 (2001) 149–168. [DOI] [PubMed] [Google Scholar]
- [51].Tabata Y, Ikada Y, Macrophage phagocytosis of biodegradable microspheres composed of Llactic acid/glycolic acid homo- and copolymers, J.Biomed.Mater.Res 22 (1988) 837–858. doi: 10.1002/jbm.820221002. [DOI] [PubMed] [Google Scholar]
- [52].Thomas C, Rawat A, Hope-Weeks L, Ahsan F, Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine, Mol.Pharm 8 (2011) 405–415. doi: 10.1021/mp100255c. [DOI] [PubMed] [Google Scholar]
- [53].Liu Y, Hardie J, Zhang X, Rotello VM, Effects of engineered nanoparticles on the innate immune system, Semin.Immunol (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Smith BR, Ghosn EE, Rallapalli H, Prescher JA, Larson T, Herzenberg LA, Gambhir SS, Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery, Nat.Nanotechnol 9 (2014) 481–487. doi: 10.1038/nnano.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Inturi S, Wang G, Chen F, Banda NK, Holers VM, Wu L, Moghimi SM, Simberg D, Modulatory Role of Surface Coating of Superparamagnetic Iron Oxide Nanoworms in Complement Opsonization and Leukocyte Uptake, ACS Nano. 9 (2015) 10758–10768. doi: 10.1021/acsnano.5b05061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wang Z, Li J, Cho J, Malik AB, Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils, Nat.Nanotechnol 9 (2014) 204–210. doi: 10.1038/nnano.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chu D, Gao J, Wang Z, Neutrophil-Mediated Delivery of Therapeutic Nanoparticles across Blood Vessel Barrier for Treatment of Inflammation and Infection, ACS Nano. 9 (2015) 11800–11811. doi: 10.1021/acsnano.5b05583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Weilbacher M, Allmeroth M, Hemmelmann M, Ritz S, Mailander V, Bopp T, Barz M, Zentel R, Becker C, Interaction of N-(2-hydroxypropyl)methacrylamide based homo, random and block copolymers with primary immune cells, J.Biomed.Nanotechnol 10 (2014) 81–91. [DOI] [PubMed] [Google Scholar]
- [59].Maharjan AS, Pilling D, Gomer RH, High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation, PLoS One. 6 (2011) e26078. doi: 10.1371/journal.pone.0026078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Oh N, Park J-H, Endocytosis and exocytosis of nanoparticles in mammalian cells, International Journal of Nanomedicine. (2014). doi: 10.2147/IJN.S26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cedervall T, Lynch I, Foy M, Berggård T, Donnelly SC, Cagney G, Linse S, Dawson KA, Detailed Identification of Plasma Proteins Adsorbed on Copolymer Nanoparticles, Angewandte Chemie International Edition. 46 (2007) 5754–5756. doi: 10.1002/anie.200700465. [DOI] [PubMed] [Google Scholar]
- [62].Bertrand N, Grenier P, Mahmoudi M, Lima EM, Appel EA, Dormont F, Lim J-M, Karnik R, Langer R, Farokhzad OC, Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics, Nature Communications. 8 (2017). doi: 10.1038/s41467-017-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Nicolete R, dos Santos DF, Faccioli LH, The uptake of PLGA micro or nanoparticles by macrophages provokes distinct in vitro inflammatory response, International Immunopharmacology. 11 (2011) 1557–1563. doi: 10.1016/j.intimp.2011.05.014. [DOI] [PubMed] [Google Scholar]
- [64].Baltazar GC, Guha S, Lu W, Lim J, Boesze-Battaglia K, Laties AM, Tyagi P, Kompella UB, Mitchell CH, Acidic Nanoparticles Are Trafficked to Lysosomes and Restore an Acidic Lysosomal pH and Degradative Function to Compromised ARPE-19 Cells, PLOS ONE. 7 (2012) e49635. doi: 10.1371/journal.pone.0049635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Peter K, Rehli M, Singer K, Renner-Sattler K, Kreutz M, Lactic acid delays the inflammatory response of human monocytes, Biochemical and Biophysical Research Communications. 457 (2015) 412–418. doi: 10.1016/j.bbrc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- [66].Santos DM, Carneiro MW, de Moura TR, Soto M, Luz NF, Prates DB, Irache JM, Brodskyn C, Barral A, Barral-Netto M, Espuelas S, Borges VM, de Oliveira CI, PLGA nanoparticles loaded with KMP-11 stimulate innate immunity and induce the killing of Leishmania, Nanomedicine. 9 (2013) 985–995. doi: 10.1016/j.nano.2013.04.003. [DOI] [PubMed] [Google Scholar]
- [67].Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM, Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool, Nat.Neurosci. 14 (2011) 1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- [68].Mildner A, Mack M, Schmidt H, Bruck W, Djukic M, Zabel MD, Hille A, Priller J, Prinz M, CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system, Brain. 132 (2009) 2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- [69].Noble BT, Brennan FH, Popovich PG, The spleen as a neuroimmune interface after spinal cord injury, J. Neuroimmunol. 321 (2018) 1–11. doi: 10.1016/j.jneuroim.2018.05.007. [DOI] [PubMed] [Google Scholar]
- [70].Qiu Y, Palankar R, Echeverria M, Medvedev N, Moya SE, Delcea M, Design of hybrid multimodal poly(lactic-co-glycolic acid) polymer nanoparticles for neutrophil labeling, imaging and tracking, Nanoscale. 5 (2013) 12624–12632. doi: 10.1039/c3nr04013e. [DOI] [PubMed] [Google Scholar]
- [71].Steinbach K, Piedavent M, Bauer S, Neumann JT, Friese MA, Neutrophils amplify autoimmune central nervous system infiltrates by maturing local APCs, J.Immunol 191 (2013) 4531–4539. doi: 10.4049/jimmunol.1202613. [DOI] [PubMed] [Google Scholar]
- [72].Pierson ER, Wagner CA, Goverman JM, The contribution of neutrophils to CNS autoimmunity, Clinical Immunology. 189 (2018) 23–28. doi: 10.1016/j.clim.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Aubé B, Lévesque SA, Paré A, Chamma É, Kébir H, Gorina R, Lécuyer M-A, Alvarez JI, Koninck YD, Engelhardt B, Prat A, Côté D, Lacroix S, Neutrophils Mediate Blood–Spinal Cord Barrier Disruption in Demyelinating Neuroinflammatory Diseases, The Journal of Immunology. 193 (2014) 2438–2454. doi: 10.4049/jimmunol.1400401. [DOI] [PubMed] [Google Scholar]
- [74].Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L, Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases, J Control Release. 235 (2016) 34–47. doi: 10.1016/j.jconrel.2016.05.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Particle association with monocytes and neutrophils in vitro. Blood cells were collected from EAE mice at 9 days p.i. and following red blood cell lysis, 200ug/mL of PLG-H PDLA, and PLG-L particles were added and incubated for 0.5, 1 and 3 hours. Representative flow cytometry plots (left) and percent (right) of (A) Ly6Chi monocytes (CD45+/CD11b+/Ly6G−/Ly6Chi), (B) Ly6Clow monocytes (CD45+/CD11b+/Ly6G−/Ly6Clow), and (C) neutrophils (CD45+/CD11b+/ Ly6G+) which were associated with particles were also calculated via gating cy5.5 positive cells. All data represent mean ± standard deviation (SD) (N = 4 per group).
Fig. S2. Clinical scores of EAE mice receiving consecutive 6 days of particle infusion. EAE mice were intravenously injected with particles (PLG-L, PLG-H and PDLA) or PBS control starting day 8 p.i. until day 13 p.i. The data plots clinical scores for PLG-H particles (red square), PDLA (blue triangle), PLG-L (green circle) and PBS (black cross).
Fig. S3. Particle association with monocytes and neutrophils in the blood. PLG-H and PDLA particles were injected to EAE mice at 10 days p.i., and then blood was collected at 1 and 3 hours after the injections. Representative flow cytometry plots of (A) Ly6Chi monocytes, and Ly6Clow monocytes, and population of (B) Ly6Chi monocytes and (C) Ly6Clow monocytes in CD45+ cells were determined. (D) Representative flow cytometry plots and population of (E) neutrophils in CD45+ cells were determined. All data represent mean ± standard deviation (SD) (N = 4 per group).
Fig. S4. (A) Population of particle positive CD45+ cells in total CD45+ cells in the blood. (B) Proportion of particle associated Ly6Chi monocytes, Ly6Clow and neutrophils in CD45+ cells in the blood.
Fig. S5. Particle association with monocytes and neutrophils in the blood. PLG-H and PDLA particles were injected to EAE mice at 24 hours after consecutive particle injections. (A) Population of Ly6Chi monocytes, Ly6Clow monocytes, and neutrophils in CD45+ cells were determined. (B) Representative flow cytometry plots (left) and population (right) of Ly6Chi monocytes, Ly6Clow monocytes, and neutrophils associated with particles were determined. All data represent mean ± standard deviation (SD) (N = 4 per group).
Fig. S6. CD4+ T-cells in the spleen. (A) Representative flow cytometry plots showing how subsets of CD4+ T-cells were gated. (B) Population of CD4+ T-cell among live cells were calculated. All data represent mean ± standard deviation (SD) (N = 4 per group).