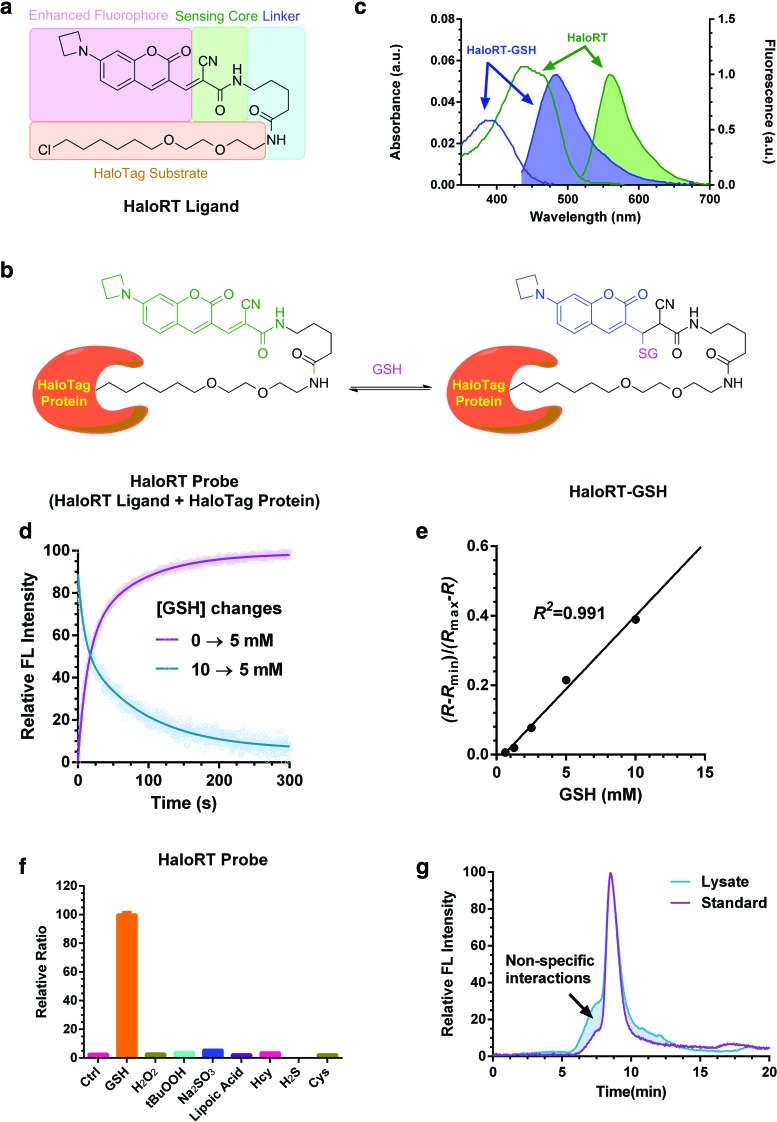
FIG. 1.
Characterization of HaloRT probe. (a) Chemical structure of HaloRT ligand and the modular design diagram. (b) Reversible reaction between HaloRT and GSH. (c) Deconvoluted, normalized UV-vis absorption spectra (unshaded) and fluorescent spectra (shaded) of HaloRT (green, λex = 488 nm) and HaloRT-GSH conjugate (blue, λex = 405 nm). (d) Reaction kinetics of HaloRT and GSH measured on a stopped-flow rapid mixing device with a fluorescence detector. Forward and backward reactions were monitored at λex = 405 nm, λem = 480 nm, which correspond to the formation and dissociation of HaloRT-GSH conjugate. (e) Calibration curve determined by mixing a series of known concentrations of GSH with HaloRT, followed by imaging on a coverslip using confocal microscopy. The calculated Kd′ is 23.7 mM, and the R2 is 0.991 in the physiologically relevant concentration range of GSH. (f) Selectivity of HaloRT probe in solution phase. Relative fluorescent ratio between blue and green channels was measured following mixing HaloRT probe with GSH (10 mM), H2O2 (100 μM), tBuOOH (100 μM), Na2SO3 (100 μM), lipoic acid (100 μM), homocysteine (100 μM), hydrogen sulfide (100 μM), and cysteine (100 μM). (g) Gel permeation chromatography trace of standard HaloRT-GSH conjugate and lysate from cyto-HaloRT-stained HeLa cells. Fluorescent signal (λex = 405 nm, λem = 480 nm) was used for detecting HaloRT-GSH conjugate. Calculation of the area under the curve revealed that 87% of the signal can be attributed to HaloRT-GSH, indicating high selectivity toward GSH over protein thiols inside cells. FL, fluorescence; GSH, glutathione; H2O2, hydrogen peroxide; RT, RealThiol probe. Color images are available online.