Abstract
The article describes the results of a retrospective analysis of medical records of 395 patients with a clinical diagnosis of leptospirosis treated at the Lviv Oblast Infectious Disease Clinical Hospital (Ukraine) between 2002 and 2016. The main risk factors for leptospirosis were contact with rodents or their excrements (26.84%) and bathing in ponds, small lakes, and reservoirs (10.63%). Among 276 patients in whom the anti-leptospira antibodies were detected by the microscopic agglutination test (MAT), the most common serotypes were Leptospira icterohaemorrhagiae (33.33%) and Leptospira grippotyphosa (25.0%). The mortality rate was significantly higher in patients where leptospirosis diagnosis was established based on clinical symptoms without confirmation by MAT (15.13% vs. 5.43%, p < 0.01).
Keywords: leptospirosis, epidemiology, Leptospira icterohaemorrhagiae, microagglutination test
Introduction
Leptospirosis is a global bacterial zoonosis with protean manifestations (Bharti et al. 2003) beginning with fever and general malaise, often progressing to involvement of renal, hepatic, pulmonary, cardiovascular, and nervous systems, with or without hemorrhagic manifestations (Levett 2001). Contaminated animal urine, tissues, or water are factors of transmission for humans. Nearly 160 mammalian species are the reservoirs and sources of infection, such as rodents, cattle, pigs, buffaloes, horses, sheep, goats, squirrels, bandicoots, and raccoons. (Zala et al. 2014). This disease is caused by different serogroups that belong to the species Leptospira interrogans. Disease incidence varies widely from 0.1 to 975 per 100,000 people and is affected by the climate, locality, and socioeconomic status of individuals. The highest incidence is in countries with tropical and subtropical climate, where seasonal rainfall and flood are typical. Approximately 1 million people in the world suffer from leptospirosis each year with about 59,000 fatal infections annually (Costa et al. 2015). Case-fatality rate for leptospirosis varies from 5% to 40% (Haake and Levett 2015). Morbidity and mortality due to leptospirosis are higher in countries where there is no epidemiological surveillance, as well as in the case of insufficient economic development with low living standards (Taylor et al. 2015).
In Ukraine, leptospirosis is a significant cause of morbidity and mortality, and case registration is mandatory. During 2002–2016, there were 7459 cases of leptospirosis registered in Ukraine, of which 495 cases (6.6%) were registered in Lviv Oblast (Lviv Region). Nationally, the incidence in this period in Ukraine was 1.07 cases per 100,000 population; however, in Lviv Oblast a high incidence of 1.27 cases per 100,000 population was reported. Most patients diagnosed with leptospirosis in Lviv Oblast are treated as inpatients at the Lviv Oblast Infectious Disease Clinical Hospital (LOIDCH). In this study, we described epidemiological, clinical, and laboratory findings in patients admitted to LOIDCH during the study period.
Materials and Methods
Patient population
We conducted a retrospective analysis of medical records of 395 patients who received inpatient treatment at LOIDCH from 2002 to 2016. The diagnosis of leptospirosis was based on data from the epidemiological history, clinical symptoms, results of routine laboratory testing, and results of microscopic agglutination tests (MAT). MAT antibody negative patients were included in the study if they met standard clinical criteria, including fewer and at least two symptoms or signs for leptospirosis (chills, headache, myalgia, conjunctival hyperemia, skin and mucous membrane hemorrhage, rash, jaundice, myocarditis, meningitis, renal failure, and respiratory symptoms such as hemoptysis), and lacked another confirmed or probable diagnosis that explained their symptoms and laboratory findings at the time of hospital discharge. Data available in the clinical record were collected, which included epidemiologic data (possible contact with rodents or their excrements, bathing in ponds, small lakes, and reservoirs, and work in plumbing, water treatment facilities, or mining, deratization, hunting, trauma from equipment, and consuming untreated food), routine laboratory test results, and clinical data. Classification of disease severity was based on the treating physician's diagnosis of mild, moderate, or severe leptospirosis documented in the discharge or death summary. Epidemiological data were collected from patients at hospital admission.
Antibody testing for leptospirosis
Serum was obtained for testing by MAT seven or more days after the onset of symptoms. Briefly, antigens consisted of 4-day-old cultures of the Leptospira strains standardized to a density of 100 or more organisms per high-power (200 × magnification) field without spontaneous agglutination or foreign particles (e.g., precipitate). A battery of 13 pathogenic serovars, representing 11 serogroups, was used. The serovars included the following: Leptospira icterohaemorrhagiae, Leptospira javanica, Leptospira canicola, Leptospira autumnalis, Leptospira australis, Leptospira pomona, Leptospira grippotyphosa, Leptospira bataviae, Leptospira tarassovi, Leptospira hebdomadis, Leptospira pyrogenes, Leptospira ballum, and Leptospira cynopteri.
Serum was inactivated at 56°C for 30 min and then diluted with normal saline (pH 7.2–7.4) starting at 1:100. For positive samples, the final titer was determined by dilution to 1:25,600. One drop (0.05 mL) of each serum dilution was added to a well of a polystyrene plate (Poliplast) followed by one drop (0.05 mL) of leptospira antigen. Plates were covered and incubated for 1 h at 37°C.
The resulting preparation, “crushed drop,” was viewed by dark-field microscopy (Microscope details, e.g., Olympus IX70 or whatever, dark-field condenser OI-13) with magnification of 20 × 10 using standard preparation glasses and 15 × 15 mm cover glasses. Microagglutination was visualized as leptospira “glueing” and as formation of “spiders,” “bows,” and “braids.” Agglutination was ranked as 1+ with 25% of the leptospires clumped, 2+ with about 50% clumped, 3+ with ∼75% clumped, and 4+ when more than 75% agglutination occurred. The end point was the highest dilution showing a 2+ reaction. The results were considered valid only in the absence of any lysis and agglutination in negative control wells. Positive and negative control sera and a mixture of cultures with normal saline at 1:1 were tested each time the test was performed.
The criteria for serological confirmation of the leptospirosis diagnosis using MAT were detection of antibodies to one of the 13 Leptospira serovars from the diagnostic kit at titer of 1:200 or more, with agglutination of 2+ or more (in the case of one serum testing) or detection of a fourfold increase in antibody titer in paired sera (provided that lysis of one of the cultures or 1+ agglutination in titer 1:100 was detected in the first serum). In the case where a patient's serum was agglutinated with two or more serovars, attribution to a serovar was determined based on the serovar with highest final titer (maximum dilution).
Statistical methods
Patient data were recorded in a single Excel database. Statistical analysis was conducted using the following methods. The significance threshold was set at 0.05. Continuous variables were summarized using mean ± standard error. Student's t-test or nonparametric Mann–Whitney U test was used for detection of statistically significant difference between groups, based on the presence or absence of the Z-distribution of data. Statistical analysis of the relative values was carried out using Fisher's exact test.
Results
Participant characteristics
During the study period, 395 people diagnosed with leptospirosis received inpatient treatment at LOIDCH, where standard inpatient treatment was with either intravenous penicillin or an intravenous cephalosporin. Among patients hospitalized with a clinical diagnosis of leptospirosis, 362 patients (91.65%) recovered and 33 (8.35%) died, and 316 were male (80.0%), and 79 (20.0%) were female, (p < 0.001). The average age of patients was 49.8 years old. The average age of male patients (46.24 years) was significantly lower compared with females (59.54 years), p < 0.01. Most, 191 (48.35%), were 18–59 years old. Rural residents were slightly more represented than city residents, 201 (50.88%) versus 194 (49.12%), respectively. Residents of cities were more likely to live in apartments (75.26%) than in private homes (24.74%), p < 0.01, whereas 99% of village residents lived in private houses. Mild disease was observed in 9 (2.28%) cases, moderate disease was observed in 108 (27.34%), and severe disease occured in 278 (70.38%). In 276 (69.87%) cases, anti-leptospira antibodies were detected by MAT (MAT+), and in 119 (30.13%) cases anti-leptospira antibodies were not detected (MAT−), p < 0.001. The mortality rate was significantly higher in MAT (−) patients (15.13%) than in MAT (+) patients (5.43%, p < 0.01) (Table 1).
Table 1.
Demographic Data on 395 Patients with Clinical Leptospirosis With and Without Detection of Antibody by the Microscopic Agglutination Test Admitted to an Infectious Diseases Inpatient Unit in Lviv, Ukraine from 2002 to 2016
| Data | Patients with (+) MAT | Patients with (−) MAT | p between MAT(+)/MAT (−) patients | ||
|---|---|---|---|---|---|
| Absolute no. | % | Absolute no. | % | ||
| No. of cases | 276 | 69.87 | 119 | 30.13 | p < 0.001 |
| Age | |||||
| Less than 18 years | 5 | 1.81 | 1 | 0.84 | p > 0.05 |
| 18–44 years | 103 | 37.31 | 40 | 33.61 | |
| 45–59 years | 97 | 35.14 | 51 | 42.85 | |
| 60–74 years | 60 | 21.74 | 21 | 17.64 | |
| 75 years or more | 11 | 3.98 | 6 | 5.04 | |
| Gender | |||||
| Male | 223 | 80.8 | 93 | 78.15 | p > 0.05 |
| Female | 53 | 19.2 | 26 | 21.85 | |
| Residence | |||||
| City | 142 | 51.45 | 52 | 43.69 | p > 0.05 |
| Village | 134 | 48.55 | 67 | 56.31 | |
| Severity of disease | |||||
| Mild | 7 | 2.54 | 2 | 1.68 | p > 0.05 |
| Moderate | 71 | 25.72 | 37 | 31.1 | |
| Severe | 198 | 71.74 | 80 | 67.22 | |
| No. of lethal cases | 15 | 5.43 | 18 | 15.13 | p < 0.01 |
Results were compared using Fisher's two-sided criterion.
MAT, microscopic agglutination test.
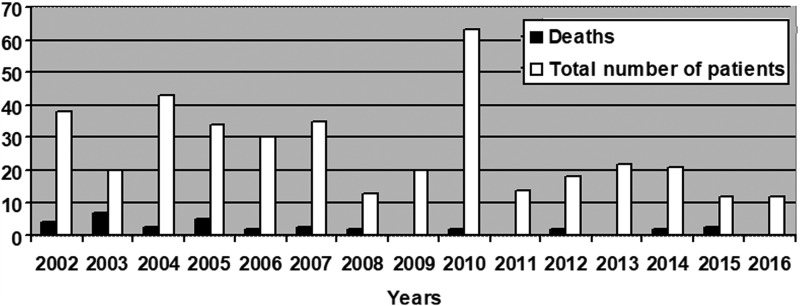
The largest number of leptospirosis cases, 63 (15.95%), were hospitalized in 2010, while only 12 cases (3.04%) were admitted in 2015–2016. The highest mortality rate, 35% (7/20), was observed in 2003, whereas no patient died in 2009, 2011, 2013, or 2016 (Fig. 1). Patients with the following occupational status were the most likely to get leptospirosis: pensioners (24.81%), manual workers (28.86%), and workers with unspecified occupation (31.9%). There was no difference in the occupation between MAT (+) and MAT (−) patients..
FIG. 1.
The number of patients with leptospirosis and the number of deaths from leptospirosis in 2002–2016 in Lviv Oblast Infectious Disease Clinical Hospital.
The epidemiological history was available for 233 patients (59.99%). Epidemiological risk factors were more likely to be present in the MAT (+) patients (64.1%) than in the MAT (−) patients (47.05%), p < 0.01. The most frequently reported risk factors among 395 patients were as follows: possible contact with rodents or their excrements in the home (26.84%), bathing in ponds, small lakes, and reservoirs (10.63%), and work in plumbing, water treatment facilities, or mining (8.61%). Less frequently, patients reported haying (4.05%) or working in a fishery (1.77%). There was no significant difference in the MAT (+) and MAT (−) patients in identification of individual epidemiological risk factors.
Serum testing
Among 276 leptospirosis patients for whom the diagnosis was confirmed using MAT, antibody to L. icterohaemorrhagiae was detected in 92 (33.33%) patients, L. grippotyphosa in 69 (25%), L. pomona in 23 (8.33%), L. canicola in 20 (7.25%), L. hebdomadis in 11 (3.99%), and L. cynopteri in 10 (3.62%). Less frequent serogroups were L. bataviae in six (2.17%), L. javanica and L. autumnalis in four (1.45%), L. sejro in three (1.09%), L. cabura in two (0.72%), and L. ballum in one (0.36%). In 31 patients (11.23%), it was not possible to establish a single dominant serogroup.
Among the 33 patients that died, the MAT was positive in 16 (48.48%), including 9 (56.25%) with antibody directed at L. icterohaemorrhagiae. In two fatal cases (12.50%), antibodies were detected to L. grippotyphosa, two (12.50%) to L. cynopteri, and two (12.50%) to L. cabura. One patient (6.25%) had antibody to both L. icterohaemorrhagiae and L. grippotyphosa. L. icterohaemorrhagiae was identified in 83 of 260 (31.92%) MAT (+) patients who survived, compared to 9 of 16 (56.25%) MAT (+) patients that died (p = 0.05).
Laboratory findings
Analyzing the laboratory parameters at hospital admission revealed no significant difference between MAT (+) and MAT (−) groups in the average level of erythrocytes, leukocytes, thrombocytes, ESR, creatinine, urea, and alanine aminotransferase (ALT) in blood. However, at admission the hemoglobin level was significantly higher in the MAT (−) group (125.4 g/L) compared to the MAT (+) group (120.25 g/L), p < 0.05. In addition, the level of total bilirubin at admission to the hospital was significantly higher in MAT (+) patients compared with MAT (−) patients, 173.67 μmol/L versus 125.36 μmol/L, respectively, p < 0.01. At discharge, a significantly higher level of hemoglobin in the MAT (−) patients was maintained, compared with the MAT (+), 117.83 g/L versus 110.49 g/L, respectively, p < 0.01.
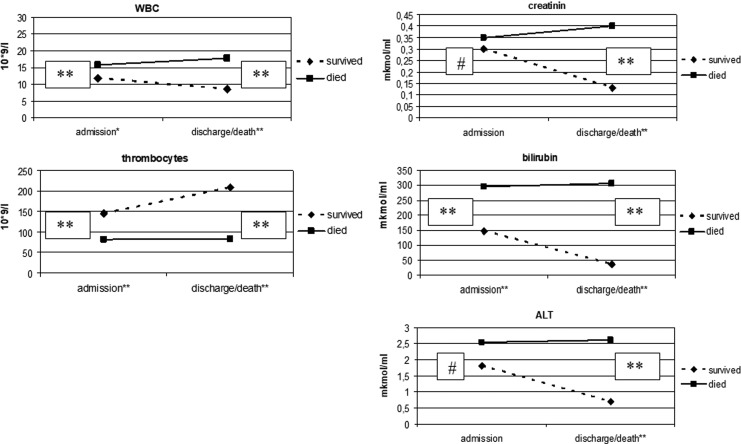
Mean values for leukocytes, thrombocytes, ALT, bilirubin, and creatinine at the time of admission and the time of discharge or death for patients who survived versus patients that died are shown in Figure 2. At the time of admission, leukocyte and bilirubin levels were significantly higher, and erythrocyte and thrombocytes were significantly lower in patients who died. At the time of discharge or death, leukocyte, creatinine, and bilirubin levels were significantly higher and erythrocyte and thrombocytes were significantly lower in patients who died. Significant differences were found in the same parameters at the time of discharge or death (Fig. 2). In addition, blood urea nitrogen was also significantly higher at the time of admission and at the time of discharge or death in those who survived versus those who died (data not shown).
FIG. 2.
Mean results of selected routine tests on the day of admission and at discharge (or death) from the hospital in patients that survived versus those that died from leptospirosis. Results were compared using the Mann–Whitney U test. #No significant difference after the group comparison; *p < 0.05 compared to indicator from the respective group; **p < 0.01 compared to indicator from the respective group; Demographic data on 395 patients with clinical leptospirosis with and without detection of antibody by the MAT admitted to an infectious diseases inpatient unit in Lviv, Ukraine from 2002 to 2016. Results were compared using Fisher's two-sided criterion. MAT, microscopic agglutination test.
Discussion and Conclusions
In this retrospective study, we describe the clinical and epidemiological characteristics of 395 cases of leptospirosis in whom the diagnosis was confirmed by MAT in 276 (69.87%) patients. In most published reports, only patients with a laboratory confirmed diagnosis were included (Katz et al. 2001, Leshem et al. 2010). Less commonly, researchers include patients with clinical signs of leptospirosis without laboratory confirmation (Ko et al. 1999).
Of note, mortality was significantly higher in patients where leptospirosis was confirmed based on clinical signs, but MAT results were negative. To our knowledge, this finding has not been reported in previous studies. Possible explanations for this difference in mortality include the following: first, in patients who died in early stages of the disease, there may not have been enough time for antibodies to form. Alternatively, some MAT (−) deceased patients could have died from other diseases such as hemorrhagic fever with renal syndrome (HFRS) or Crimean-Congo hemorrhagic fever (CCHF) that have similar presentations (Markotić et al. 2002, Golubić and Markotić 2003, Agampodi et al. 2011, Seifi et al. 2016).
According to the literature, leptospirosis severity and mortality is higher in cases where the causative agent is L. icterohaemorrhagiae (Leshem 2010, Tubiana et al. 2013). We have found the same result. In majority of fatal cases that were laboratory confirmed, this serogroup caused the disease.
It should be mentioned that leptospirosis mortality rate has a wide range; 15–17% case fatality rate was reported in studies from Brazil (Ko et al. 1999, Spichler et al. 2008). In research that was conducted in Greece, lethality was 30% (Velissaris et al. 2012). In another study conducted in India, lethality was 52% (Chawla et al. 2004). In our study the lethality rate is 8.35%, which is significantly lower compared to the mortality rates from leptospirosis reported from other countries.
One of the strengths of our study is that it included significant number of patients, 395, studied over a long period (2002–2016). All patients were treated in the same hospital, which provided the same approach to the diagnosis and treatment for patients, including access to the same critical care unit. Blood from all patients was tested for antibodies in a single reference laboratory using the same assay throughout studying period.
A limitation of the study is that the retrospective design limited our information about patients to data available in routine medical records, including the treating physician's assessment of disease severity. Another limitation of this work is the low specificity and sensitivity of MAT during the initial 7–14 days of disease used for the confirmation of leptospirosis in our patients since the specificity and sensitivity of MAT peaks at the third or fourth week after the symptom onset (Veerappa Budihal et al. 2014, Niloofa et al. 2015). However, even when samples from the third or fourth week are available for testing by MAT, the specificity and sensitivity were not 100% (Cumberland et al. 1999, Di Limmathurotsakul et al. 2012). It is also impossible to exclude the possibility of cross-reactions using the MAT that could potentially influence the accuracy of identification of particular serological group of leptospira. It is also possible that we detected of pseudo-positive reactions of MAT through cross-reacting antibodies to other spirochetes, including Lyme borreliosis and syphilis. Unfortunately, during the study period, we did not have the PCR method to confirm the diagnosis of leptospirosis.
We are encouraged by the fact that PCR testing of urine and blood for leptospira became available for our hospital at the end of 2016. Testing of urine samples using PCR gives positive results in urine within the first week of the disease when MAT results are still negative (Bhatia and Umapathy 2015). The literature suggests that the combination of MAT and PCR for diagnosis of leptospirosis is considered the gold standard (Agampodi et al. 2016). We hope that the use of this technique will increase the frequency of specific identification of the diagnosis of leptospirosis in the early stages of the disease. We also hope that we will be able to implement the use of specific diagnostics for viral diseases such as HFRS and CCHF.
Acknowledgments
The authors express their gratitude to Defense Threat Reduction Agency and Ukraine Biological Threat Reduction Program for the support in publication of this article. Authors are thankful to Dr. Gregory Mertz (University of New Mexico Albuquerque, NM) for his mentoring of this article. Authors also thank the Archive of LOIDCH for providing access to medical records of patients diagnosed with leptospirosis and the State Institution Lviv Oblast Laboratory Center of the Ministry of Health of Ukraine for conducting MAT.
Author Disclosure Statement
The authors acknowledge the United States Department of Defense, Defense Threat Reduction Agency (DTRA), and Cooperative Biological Engagement Program (CBEP) for their support to develop this article. While DTRA/CBEP did not support the research described in this publication, the Program supported the presentation of this research in an international forum and supported grantsmanship/science writing training related to the development of this article. The contents of this publication are the responsibility of the authors and do not necessarily reflect the views of DTRA or the United States Government.
References
- Agampodi B, Dahanayaka J, Nöckler K, Mayer-Scholl A, et al. Redefining gold standard testing for diagnosing leptospirosis: Further evidence. Am J Trop Med Hyg 2016; 95:531–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agampodi B, Peacock J, Thevanesam V, Nugegoda B, et al. Leptospirosis outbreak in Sri Lanka in 2008: Lessons for assessing the global burden of disease. Am J Trop Med Hyg 2011; 85:471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti AR, Nally JE, Ricaldi JN, Matthias MA, et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect Dis 2003; 3:757–771 [DOI] [PubMed] [Google Scholar]
- Bhatia M, Umapathy B. Deciphering leptospirosis a diagnostic mystery: An insight. Int J Med Res Health Sci 2015; 4:693–701 [Google Scholar]
- Chawla V, Trivedi T, Yeolekar M. Epidemic of leptospirosis: An ICU experience. J Assoc Phys India 2004; 52:619–622 [PubMed] [Google Scholar]
- Costa F, Hagan José, Calcagno J, Kane M, et al. Global morbidity and mortality of leptospirosis: A systematic review. PLoS Negl Trop Dis 2015; 9:e3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberland P, Everard CO, Levett PN. Assessment of the efficacy of an IgM-ELISA and microscopic agglutination test (MAT) in the diagnosis of acute leptospirosis. Am J Trop Med Hyg 1999; 61:731–734 [DOI] [PubMed] [Google Scholar]
- Golubić D, Markotić A. Leptospirosis and hemorrhagic fever with renal syndrome in northwestern Croatia. Acta Med Croatica 2003; 57:369–372 [PubMed] [Google Scholar]
- Haake D, Levett P. Leptospirosis in humans. Curr Top Microbiol 2015; 387:65–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A, Ansdell V, Effler P, Middleton C, et al. Assessment of the clinical presentation and treatment of 353 cases of laboratory confirmed leptospirosis in Hawaii, 1974–1998. Clin Infect Dis 2001; 33:1834–1841 [DOI] [PubMed] [Google Scholar]
- Ko AI, Galvão Reis M, Ribeiro Dourado CM, Johnson WD Jr, et al. Urban epidemic of severe leptospirosis in Brazil. Salvador Leptospirosis Study Group. Lancet 1999; 354:820–825 [DOI] [PubMed] [Google Scholar]
- Leshem E, Segal G, Barnea A, Yitzhaki S, et al. Travel-related leptospirosis in Israel: A Nationwide Study. Am J Trop Med Hyg 2010; 82:459–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levett PN. Leptospirosis. Clin Microbiol Rev 2001; 14:296–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limmathurotsakul B, Turner E, Wuthiekanun V, Suputtamongkol Y, et al. Fool's gold: Why imperfect reference tests are undermining the evaluation of novel diagnostics: A reevaluation of 5 diagnostic tests for leptospirosis. Clin Infect Dis 2012; 55:322–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markotić A, Kuzman I, Babić K, Gagro A, et al. Double trouble: Hemorrhagic fever with renal syndrome and leptospirosis. Scand J Infect Dis 2002; 34:221–224 [DOI] [PubMed] [Google Scholar]
- Niloofa R, Fernando N, Lakshitha N, Karunanayake L, et al. Diagnosis of leptospirosis: Comparison between microscopic agglutination test, IgM-ELISA and IgM rapid immunochromatography test. PLoS One 2015;10:e0129236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifi A, Hajiabdolbaghi M, Mohammadnejad E. Co-infection of leptospirosis and crimean-congo hemorrhagic fever. Arch Clin Infect Dis 2016; 11:e32380 [Google Scholar]
- Spichler S, Vilaça J, Athanazio A, Albuquerque O, et al. Predictors of lethality in severe leptospirosis in urban Brazil. Am J Trop Med Hyg 2008; 79:911–914 [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Paris D, Newton P. A systematic review of the mortality from untreated leptospirosis. PLoS Negl Trop Dis 2015; 9:e0003866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubiana S, Mikulski M, Becam J, Lacassin F, et al. Risk factors and predictors of severe leptospirosis in New Caledonia. PLoS Negl Trop Dis 2013; 7:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerappa Budihal S, Perwez K. Leptospirosis diagnosis: Competancy of various laboratory tests. J Clin Diagn Res 2014; 8:199–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velissaris D, Karanikolas M, Flaris N, Fligou F, et al. Commonly used severity scores are not good predictors of mortality in sepsis from severe leptospirosis: A series of ten patients. Crit Care Res Pract 2012;2012:532376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala DB, Khan V, Das VK. A study on few biochemical parameters of clinically suspected and laboratory confirmed Leptospirosis cases. J Appl Nat Sci 2014; 6:12–13 [Google Scholar]