Abstract
Vision and audition provide spatial information about the environment to guide natural behavior. Because the eyes move in the head while the ears remain head-fixed, input conveying eye position in the head is required to maintain audiovisual congruence. Human perception of auditory space was previously shown to shift with changes in eye position, regardless of the target’s frequency content and spatial cues underlying horizontal and vertical localization. In this study, we examined whether this interaction is altered by advancing age. Head-restrained young (18–44 yo), middle-aged (45–64 yo), and elderly (65–81 yo) human subjects localized noise bursts under conditions of transient and sustained ocular deflection. All three age groups demonstrated a time-dependent shift of auditory space in the direction of eye position. Moreover, this adaptation showed a clear decline with advancing age, but only for peripheral auditory space (beyond ±10° from midline). Alternatively, adaptation in the periphery may occur, but is more sluggish than in the central field and therefore not fully observed in this experiment. The age-dependent effect cannot be readily explained by senescent peripheral hearing loss, suggesting a change in central processing of auditory space in relation to the control of gaze.
Keywords: Aging, Sound localization, Eye movements, Sensory integration, Spatial processing, Egocentric perception
Introduction
Vision and audition provide spatial information relevant to the environment during natural daily activities. Since the retinae move with the eyes while the ears remain head-fixed, information about eye position in the head is required to maintain spatial congruence between the two senses, presumably by transforming audition and vision onto a common coordinate scheme and reference frame. Interestingly, previous behavioral studies in humans and non-human primates have demonstrated that sound localization shifts in response to changes in eye position (Bohlander 1984; Getzmann 2002; Lewald 1997, 1998; Lewald and Ehrenstein 1996, 1998, 2000; Lewald and Getzmann 2006; Metzger et al. 2004; Weerts and Thurlow 1971; Yao and Peck 1997). However, the magnitude, direction, and uniformity of the shift reported in these studies vary widely, likely reflecting differences in methodology.
Recently, Razavi et al. (2007) demonstrated in young subjects that auditory spatial perception adapts to prolonged changes in eye position by shifting in the direction of ocular eccentricity in an exponential manner, approaching ~40% of sustained eye eccentricity over several minutes. Because the adaptation begins immediately following a change in eye position, it biases sound localization even within the few seconds required for subjects to fixate and point to a target. The resulting overshoot in auditory localization that occurs when eye movements are used to guide responses declines by ~70% when the eyes are held stationary in line with the head and peripheral vision is used to guide pointing. Moreover, adaptation requires no visual input, extends to all auditory spatial channels carrying interaural disparity and spectral cues, and is accompanied by equal or greater shifts in the perception of straight-ahead (Cui et al. 2010). By contrast, localization from memory of transiently presented visual targets is not similarly affected.
In this study, we examined whether the adaptation of auditory space by changing eye position is affected by advancing age. This question has implications for the well-known disequilibrium that often afflicts the elderly; deterioration of spatial-sensory function can have severe consequences such as injury from falls. Such age-dependent change might arise due to changes in learning and/or processing of central interactions between auditory space and eye movements. The former is salient in that presbycusis, or age-related hearing loss, is classically characterized by bilateral loss of auditory sensitivity that progresses from high to low frequencies, and often involves deficits in speech recognition and central auditory processing (Frisina and Frisina 1997; Gates et al. 1990; Schuknecht 1964). In spatial perception, localization accuracy for broadband targets in the elderly is comparable to younger subjects in horizontal/azimuthal (Az) space, albeit with increased front/back confusion and reduced precision (Abel et al. 2000; Abel and Hay 1996). In contrast, because high-frequency hearing is crucial for vertical/elevation (El) discrimination, localization in El is greatly impaired in presbycusic individuals compared to younger subjects (Dobreva et al. 2005; Noble et al. 1994; Rakerd et al. 1998).
Given the profound effect of eye position on spatial perception in younger subjects, we directly addressed whether aging alters the adaptation of auditory space to changes in eye position, and if so, whether a relationship exists between these age-related differences and hearing loss. Further, we also quantified perceived straight-ahead (PSA) in the same individuals in order to assess whether potential age-dependent alteration in the influence of eye position extends to non-auditory spatial perception as previously demonstrated in young subjects (Cui et al. 2010).
Methods
Subjects
Thirty human subjects were parsed into three age groups; Young (18–39 years of age: 26.7 ± 6.5 yo, mean ± SD; 6F, 5 M), Middle-aged (40–65 years of age: 53.8 ± 5.6 yo; 5F, 4 M), and Elderly (66–81 years of age: 73.9 ± 5.6 yo; 6F, 4 M). All subjects were in good general health, with no history of sensory or motor dysfunction. Best corrected visual acuity was 20/20 or better binocularly in the Young and Middle-aged, and at worst 20/40 in the Elderly, with normal stereo-acuity (Titmus Test) and visual fields (Amsler grid and/or tangent screen). Standard videonystagmography demonstrated normal oculomotor and vestibular function (including caloric tests). Audiometric thresholds were assessed from 0.25 to 12 kHz, and at frequencies ≤4 kHz all subjects fell within the clinically normal range (≤20 dB HL). Speech-perception-in-quiet scores were between 88 and 100% on the NU-6 word recognition test.
The study was performed with approval from the University of Rochester Research Subjects Review Board in accordance with the 1964 Declaration of Helsinki. All subjects gave informed consent and were compensated for their participation.
Experimental apparatus and stimulus characteristics
Experiments were conducted in a fully enclosed, echo-attenuated 3.0 × 3.7 × 2.7 m (L, W, H) room. The chamber walls, ceiling and floor are lined with 3-thick SonexTextile acoustic panels (Illbruck, now Pinta Acoustic), which have an acoustic absorption coefficient ≥0.99 above 250 Hz; higher frequencies were further attenuated by synthetic fleece on the apparatus surrounding the subject. Echoes were measured and found to be indistinguishable from ambient background noise for frequencies below 250 Hz (between 100 and 250 Hz). Subjects sat in complete darkness facing one corner of the room. The head was fixed with a custom bite-bar and positioned such that the midpoint between the two eyes was aligned with the geometric center of a cylindrical screen of acoustically transparent black speaker cloth at 2 m distance (Fig. 1a). Reid’s baseline, a line extending from the inferior margin of the orbit to the superior border of the external auditory meatus, was aligned with the target space horizontal plane.
Fig. 1.
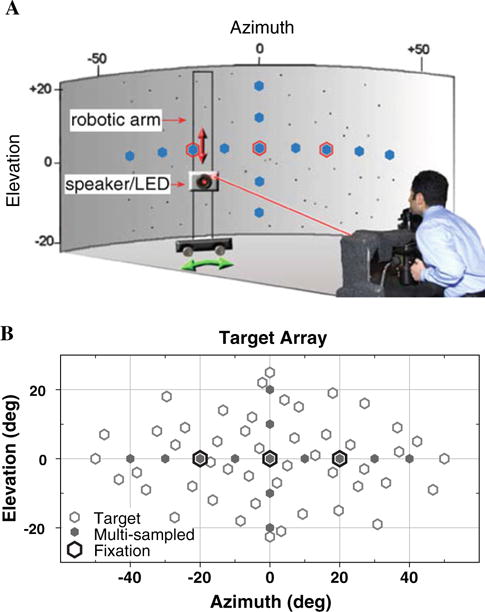
Experimental setup. a Subject position and laboratory configuration. b Target array of 61 locations within ± 50° Az by ±25° El, including 13 multi-sampled locations at 10° intervals (5 trials per session). This appears in similar fashion in a; filled symbols signify multi-sampled targets. Fixation spots appear as larger open symbols in both a and b. (Reproduced with permission from the Journal of Neuroscience, © Society for Neuroscience)
To control eye position during trials requiring sustained fixation, three red laser-LEDs projected fixation spots (≈0.1° subtended angle) onto the cylindrical screen at center (Ctr;0° Az, 0° El), and ±20° left and right of Ctr (L and R20°).
Auditory targets were presented by a single 8-cm-diameter, 2-way coaxial loudspeaker (Blaupunkt PCx 352; Hildesheim, Germany). Visual targets (≈0.1° subtended angle) were presented by a miniature red LED mounted at the center of the speaker visible through the translucent cylindrical screen. The speaker/LED assembly was mounted on a two-axis servo-controlled robotic arm hidden behind the screen, which enabled rapid and precise positioning of the target stimuli in cylindrical coordinates (Fig. 1a).
All auditory stimuli were synthesized digitally using SigGenRP software and presented using TDT System II hardware (Tucker-Davis Technologies, Alachua, FL). Auditory targets consisted of 75 dB SPL (equalized; spectrum level N0 = 32 dB SPL) broadband (0.1–20 kHz) Gaussian white noise presented in 150 ms bursts (rise/fall time = 10 ms) repeating at 5 Hz throughout a trial. Visual stimuli similarly consisted of 150 ms LED flashes repeating at 5 Hz.
Two tactics were employed to avoid the potential for subjects to predict target location. First, the robotic arm repositioned the speaker/LED in two steps of pseudorandom duration to reach the intended target position so as to avoid temporal cues. Second, Gaussian white noise (65 dB SPL) was presented from two widely separated and unseen stationary loudspeakers (Boston Acoustics CR67) between trials to mask mechanical noise emanating from the robotics.
Response measures
Visually guided pointing
In all experiments, subjects localized targets by manually aiming the beam of a laser pointer. The pointer was mounted on a 2-axis cylindrical joystick beneath the platform holding the bite-bar (Fig. 1a) and projected a spot that subtended <0.03° on the screen. Optical encoders registered joystick position in Az and El (resolution <0.1°). For each target presentation, response endpoint was registered with a key press by the subject (response time was typically ~4 s), and the target and pointer positions were recorded. No feedback on response performance was provided to the subject.
Visual calibration
At the end of each experiment, subjects localized an array of visual targets at 10° intervals within the same range as the auditory targets. This procedure served to ensure system performance and verify the subjects’ ability to perform the task.
Experimental protocol
Sound localization was assessed at 61 locations (Fig. 1b) over a range of ±50° Az by ±25° El in frontal space, including a subset of multi-sampled targets (5 trials per session) positioned at 10° intervals along the primary meridians (9 in Az and 5 in El). In all cases, the termination of the masking noise and subsequent presentation of the auditory stimulus signaled the start of a trial, during which the stimulus was presented continuously. Subjects were instructed to align the beam of the laser pointer with the perceived sound location quickly but as accurately as possible and to signal their localization endpoint with a key press. This task was easily and reliably performed by all subjects and constituted a reasonably natural means of orienting and pointing to external targets. The repositioning of the speaker accompanied by the masking noise followed immediately during the inter-trial interval. The masker was switched off once the speaker reached its new target location, and the next trial began without delay. Each experimental session was divided into two sets with a short intervening break. Two auditory localization paradigms were utilized:
Target fixation
This baseline condition exploits the natural human tendency to attend to a sound source of interest by fixating the eyes on the perceived target location. Subjects guided the laser pointer with the aid of foveal vision so as to align both the eyes and the projected spot with the perceived sound position (totaling 113 trials).
Sustained fixation
To characterize the effect of sustained eye position on sound localization across age groups, subjects maintained fixation on one of three fixation spots located at Ctr, L20° and R20° (in separate sessions) and guided the pointer to target locations using peripheral vision. During eccentric fixation, to ensure that the laser pointer remained clearly visible, target locations beyond 30° Az contralateral to fixation were excluded.
The subjective perception of straight-ahead (PSA) was also assessed. At the beginning and end of each session, and after every ~20 trials of auditory localization (8 trials per session), subjects were asked to maintain gaze on the fixation spot, draw an imaginary line straight-ahead from the tip of their nose to the screen, and align the pointer with this location.
Subjects participated in three experimental sessions of 121 or 110 trials for Ctr and eccentric fixations, respectively. In each session, subjects maintained fixation for a total of 15–20 min (110 or 121 trials, 8–10 s per trial including speaker movement).
Task monitoring
In a previous study utilizing the same experimental paradigm (Razavi et al. 2007), we found that subjects had little trouble maintaining eccentric gaze for long periods, and only rarely and briefly broke fixation. More importantly, we carefully inspected the electrooculography (EOG) records off-line and found that the average shift magnitudes for the three trials before and after breaks in fixation were within 1 standard deviation (SD) of each other. Thus, the overall auditory spatial shift remained effectively unaltered by brief eye movements intruding on steady fixation. Nevertheless, to ensure that subjects maintained the intended ocular eccentricity while performing the task, eye position was monitored by EOG at all times during spatial localization trials. Inadvertent eye movements were noted by the experimenter, and subjects were reminded to maintain fixation on the reference spot. Real-time tracking of the laser pointer and speaker positions also ensured that subjects were performing the tasks correctly.
Data acquisition and analysis
Experiments were controlled by customized software written in Visual C++ (Microsoft, Inc.), communicating directly with the robotic arm (target positioning), TDT System II (stimulus presentation), and the fixation lasers. An Excel (Microsoft, Inc.) spreadsheet served as the operator interface and stored all data.
Data were analyzed in Matlab (The MathWorks, Inc.), Excel (Microsoft, Inc.), and SYSTAT statistical software. Response accuracy (signed pointer error relative to target position) and precision (SD of accuracy) for each multi-sampled target were sorted by fixation condition (i.e., target and sustained fixation at Ctr, R20° and L20°). To remove idiosyncratic leftward or rightward subject bias during sustained fixation, values for L20° and R20° sessions were offset by the average pointer error during Ctr fixation, which served as a zero reference for each subject. The slope of the linear regression of response vs. target locations was used to quantify spatial gain (SG) across target positions: SG = 1.0 represents perfect performance, while values greater and less than 1.0 indicate over- and undershoot, respectively. To obtain Shift Magnitude (see Fig. 3a), we subtracted the SG from each individual data set on a session by session and subject by subject basis, thereby effectively nullifying the influence of gain. This analytical step prevents artifact (e.g., demonstrating a shift when none is present) and focused the analysis on shift magnitude alone (see Cui et al. 2010 for details).
Fig. 3.
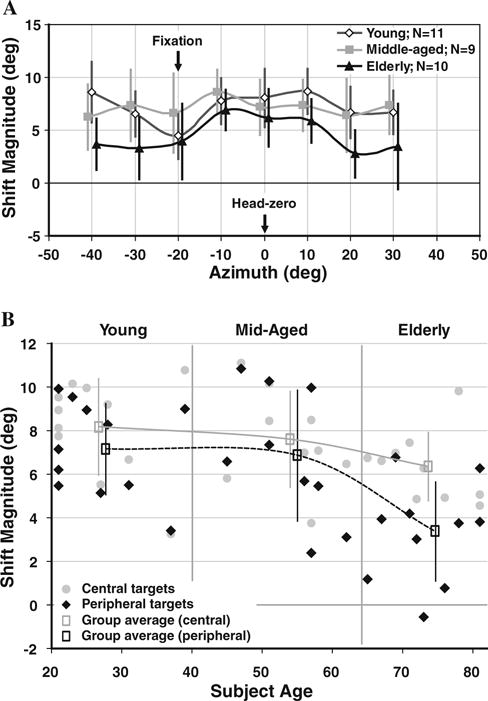
a Localization accuracy in Az during eccentric fixation (L and R) pooled within age groups and adjusted with respect to eye position such that foveal fixation is at −20° and positive auditory space is contralateral to fixation for all eccentric fixation conditions. Shift magnitude (error) is significantly lower in peripheral auditory space in the Elderly than in the other age groups. b During sustained eccentric (L and R) fixation, Az shift magnitude declines more with increasing age for peripheral targets (beyond ±10° of zero and excluding the fixation point at −20°) than for central targets (midline ±10°). The age-related decline in the shift magnitude of peripheral, but not central, auditory targets occurs in a progressive fashion; within age group averages (open rectangle) serve to emphasize the tread; values are offset for clarity; error bars indicate SD across subjects
The algebraic average (mean) and SD were calculated across subject populations for all summary statistics. Comparisons between data sets were quantified using standard parametric statistics (Student’s t-test and ANOVA), and whenever possible (i.e., within age groups), employed paired differences within subjects.
Results
Changes in spatial gain with age
In the target fixation paradigm, when subjects moved their eyes to guide the laser pointer to localize targets, horizontal localization accuracy proved comparable between age groups (ANOVA, P > 0.20; Fig. 2). All age groups demonstrated an overshoot of target position, with SG averaging 1.16 ± 0.10 (mean ± SD) for the Young, 1.13 ± 0.12 for Middle-aged, and 1.21 ± 0.13 for Elderly subjects (P = 0.50 between all age groups).
Fig. 2.
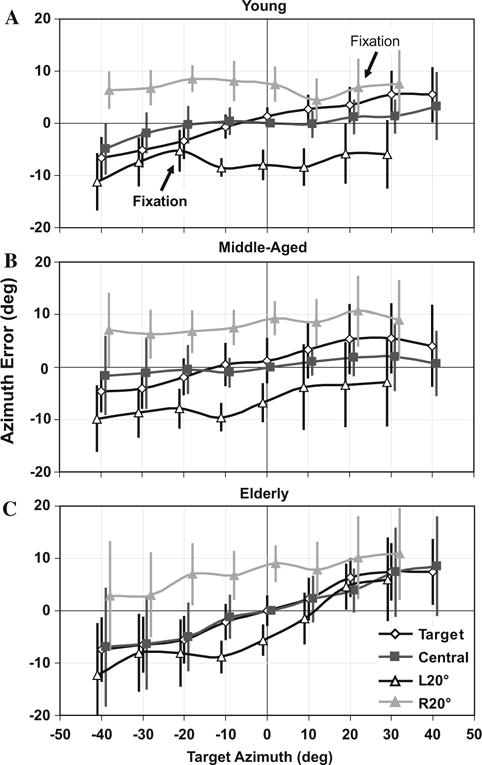
Localization accuracy (signed error between response and target location) in Az for multi-sampled targets in the horizontal plane (0° El) for Young (a), Middle-aged (b), and Elderly (c) subjects for the target fixation paradigm, and the Ctr, L20° and R20° sustained fixation paradigm. Data points are offset slightly in Az for graphic clarity. The slope of accuracy vs. target location is related to spatial gain (SG = slope ? 1.0, where 1.0 represents perfect performance). All age groups demonstrated comparable overshoot (Az SG > 1.0, or a positive slope as shown here) during target fixation. However, the overshoot is reduced significantly with Ctr fixation in the Young and the Middle-aged, but not in the Elderly. Eccentric fixation (L and R 20°) predominantly shifts perceived target Az in the direction of gaze across space
In contrast, localization in El was underestimated (SG < 1) in all age groups during target fixation. Localization undershoot in the Young was relatively modest (~10%; SG = 0.90 ± 0.21), but was significantly greater (~44%; ANOVA with Tukey HSD, P < 0.05) for both the Middle-aged (SG = 0.56 ± 0.29) and Elderly (0.56 ± 0.40). This substantial compression of vertical space perception in the two older groups was not surprising, as vertical sound localization is only reliable for spectrally complex sounds with frequency content above 4 kHz (Butler and Humanski 1992; Hebrank and Wright 1974; Roffler and Butler 1968), and both older age groups suffered diminished sensitivity at and above this frequency that likely impeded perception of important elevation cues (Noble et al. 1994).
During prolonged Ctr fixation in the sustained fixation paradigm, Young (SG = 1.07 ± 0.12) and Middle-aged (SG = 1.04 ± 0.13) subjects demonstrated ~8% reduction in Az SG compared to target fixation (Fig. 2a and b; Paired t-test, P< 0.05). This decrease in gain has been demonstrated previously (Razavi et al. 2007) and reflects the phenomenon by which changes in eye position adapt auditory space in the same direction of, and proportionally with, ocular eccentricity, even during the relatively brief time (~4 s) required to complete each trial of the target fixation task. This effect manifests as augmented SG when the eyes are used to guide sound localization and a relative reduction in SG when the eyes are fixed. Interestingly, sustained fixation in the Elderly did not result in a significant reduction in SG (<1% less than target fixation; SG = 1.20 ± 0.17; P = 1.00; Fig. 2c). Thus, the Elderly showed substantial overshoot regardless of whether the eyes were fixed or free to move. The fact that sound localization accuracy in the Elderly does not benefit from ocular fixation could also be interpreted as an age-related increase in SG compared to both younger groups.
With eccentric fixation (L and R20°), there was no significant difference in Az SG within a given age group compared to Ctr fixation (P > 0.05). In contrast to Young and Middle-aged subjects, SGs differ between L and R fixation paradigms in the Elderly, averaging 1.26 ± 0.17 and 1.13 ± 0.17, respectively (P ≤ 0.05). This asymmetry has not been seen previously and remains an interesting, albeit unexplained finding. Across age groups Az SG was greater in the Elderly (1.20 ± 0.18) than the Middle-aged (1.07 ± 0.15) and Young (1.04 ± 0.12) subjects, the difference being significant between the Young and the Elderly (P< 0.05).
Likewise, El SG remained about the same within age groups across fixation conditions, but whereas SG for the Middle-aged (0.59 ± 0.27) and Elderly (0.50 ± 0.32) were similar, it was only 50–60% of SG in the Young (0.94 ± 0.21; ANOVA with Tukey HSD, P < 0.05). These values are comparable to those seen in target fixation conditions, as discussed previously.
Auditory spatial shift in the elderly during eccentric fixation declines in the auditory periphery
Razavi et al. (2007) previously demonstrated in young subjects that SG is relatively constant for prolonged central or eccentric fixation and that the spatial adaptation to eye position applies across a wide field. Here, we confirmed that observation in the Young, but also showed that SG is relatively constant across fixation conditions in Middle-aged and Elderly subjects (Fig. 2). Therefore, to examine whether age affects the magnitude of the shift in auditory spatial perception in isolation, we normalized each data set for each subject by subtracting the regression slopes of response vs. target position to eliminate the effect of individual differences in SG (see Cui et al. 2010). Analysis was limited to Az for these experiments to avoid the influence of overall poorer performance in El.
During sustained eccentric fixation, all age groups exhibited wide-field localization shifts in the direction of eye position. However, asymmetries across the target field and localized spatial distortions in shift magnitude (changes in localization accuracy) were evident with increasing age (Fig. 2). Precision as a function of azimuth and age has been examined in a separate set of experiments conducted in our laboratory (Dobreva 2010). Briefly, precision in the young was found to be 2.3° for central targets and increased with a slope of 1.03 toward the spatial periphery for both target and Ctr fixation paradigms. In comparison, elderly subjects demonstrated a similar precision of 2.7° for central targets that increased with slopes of 1.04 and 1.1 toward the spatial periphery during target and Ctr fixations, respectively. Thus, localization precision does decrease with age under laser pointing guided by peripheral vision, though this does not affect our findings regarding localization accuracy.
To further define age-related discrepancies in localization across target space, Az localization accuracy (shift) during L and R fixation was adjusted for analysis with respect to eye position by transposing the data across the midline so that the foveal fixation angle is assigned an azimuth of −20°, and positive auditory space is therefore always contralateral to gaze (Fig. 3a). First, note the attenuated shift near the locus of ocular fixation, particularly in the young (Figs. 2a, 3a; arrows). This reduction presumably reflects visual capture, a correlate of the familiar ventriloquism effect (Alais and Burr 2004; Battaglia et al. 2003; Recanzone 1998), and refers to the process by which nearby auditory targets are drawn to the visual fixation point, thereby attenuating the shift magnitude around the fixation point. The drop in shift magnitude between 10° and 20° during rightward fixation at 20° (Fig. 2a) appears to be an idiosyncrasy of the population. Fixation was monitored via EOG at all times and was noted to be at 20° (see Methods), voiding that potential explanation. This particular aspect remains an oddity.
Second, within age groups, apart from the attenuating effect of visual capture in the region of fixation, Young and Middle-aged subjects demonstrated localization shifts that are generalized and comparable in magnitude across all Az locations (ANOVA; P > 0.15). In contrast, the auditory spatial shift in the Elderly proved less consistent across space and was significantly less in peripheral (beyond ±10° and excluding the fixation spot at −20°) when compared to central (within and including ±10°) auditory space with respect to the midline (P < 0.05; see Fig. 3b). Specifically, shift magnitude for central targets, and for targets coinciding with ocular fixation, proved indistinguishable between age groups (8.16 ± 2.25, 7.58 ± 2.24, and 6.32 ± 1.60 for Young, Middle-aged, and Elderly, respectively; P > 0.17). In contrast, shift magnitude for peripheral targets in the Elderly (3.31 ± 2.32) was significantly less than the Young (7.14 ± 2.14; P < 0.01) and Middle-aged groups (6.85 ± 3.06; P < 0.05). The fact that visual capture is less noticeable in the older age groups might also be related to the finding that the spatial shift in response to sustained eye position is attenuated for peripheral auditory targets, thus effectively obscuring the “recovery” of shift magnitude at large eccentricities.
Perceived straight-ahead (PSA) shifts during sustained eccentric fixation across age groups
PSA, like sound localization, shifted in the direction of eye position for all age groups during sustained fixation, averaging 3.16° ± 6.70°, 10.43° ± 7.93°, and 7.27° ± 6.55° for Young, Middle-aged, and Elderly subjects, respectively (AVOVA, P ≥ 0.10 between groups). Because the assessment of PSA constitutes measurement of a head-referenced spatial midline, it is most directly comparable to localization of the multi-sampled auditory target at 0° Az, 0° El (i.e., auditory spatial center). While the shift in PSA was comparable to that of auditory spatial center in the Middle-aged and Elderly (7.17 ± 2.64 and 6.18 ± 2.72, respectively; P ≥ 0.27), it proved significantly greater in the Young (8.06 ± 2.75; Tukey HSD, P< 0.05).
Discussion
The aim of our study was to examine the effect of prolonged, sustained eye position on sound localization as a function of natural aging. We employed a localization technique to orient toward and point to auditory and visual targets that has been established as precise and reliable (see Razavi et al. 2007; Cui et al. 2010). The task provided an intuitive and natural means of localizing targets, effectively similar to using a laser pointer during a presentation or a PC mouse. The effect of eye position on both sound localization and the perceived straight-ahead was quantified in adults across a wide age range (18–81 yo). A baseline for sound localization was first obtained during a target fixation task, where eye movements were free to guide the laser pointer to align both the eyes and the projected spot with the perceived target position. The eyes were then fixed on-center (0°,0°), or eccentrically ([−20°,0°] and [20°,0°]), and peripheral vision was used to guide the pointer during the sustained fixation paradigm.
In general, accuracy in Az was comparable across all age groups. Subjects overestimated target positions in the horizontal plane during target fixation when the eyes were free to guide the laser pointer. During sustained fixation, when eye position was fixed and auditory targets were localized with peripheral vision, SG in Az was significantly reduced from that during target fixation, but only in the Young and Middle-aged groups.
We have postulated previously that the eye movements used to guide localization during target fixation constitute transient changes in fixation, and as such generate a small shift in auditory space in the direction of gaze (Razavi et al. 2007). Because the shift is proportional to the magnitude of eye movement, the effect would manifest as an increase in SG (overshoot) during the target fixation task. This transient effect of eye movement has been quantified in young subjects and accounts for ~70% of the difference in SG between target and Ctr fixation tasks (Razavi et al. 2007).
Elderly subjects surprisingly showed little or no reduction in SG between target and sustained fixation tasks compared to that in the Young and Middle-aged groups. Moreover, there is an augmentation of SG with aging during sustained fixation; by correlating Az SG during sustained fixation and subject age, the increase was particularly evident beginning between 50 and 60 years of age (data not shown). This suggests that compared to the younger groups, the overshoot in the Elderly during target fixation is less attributable to the effect of eye movement alone. In combination, these changes in SG indicate an age-related attenuation in the effect of eye position on auditory spatial perception, though other results argue for a more nuanced interpretation.
Although auditory spatial perception shifted in the direction of sustained eccentric fixation regardless of age, the shift proved less uniform across auditory space in the Elderly than in other age groups. Peripheral targets were less affected than central ones (within and including ±10°), indicating an adaptive process that favors central over peripheral head-centered (as opposed to eye-centered) space. Alternatively, it is possible that adaptation of peripheral auditory space in the Elderly is governed by a slower temporal process than that affecting central auditory space, and that given sufficient time, the peripheral shift might approach that seen centrally. Indeed, the fact that the Elderly demonstrated similar overshoot regardless of whether the eyes were fixed or free to move might support the notion that the adaptation of auditory space occurs with a reduced tempo in aging. We can test these hypotheses in the future by comparing the adaptive shift of central and peripheral targets during sustained fixation over separate sessions; the shift magnitude of peripheral targets should continue to approach that of central targets over time if the tempo of the adaptive process differs between the two regions of space in the Elderly.
It is worth noting that the differences in SG and shift magnitude between Young, Middle-aged, and Elderly subjects are unlikely to be a result of visual and/or auditory sensory decline in aging. In separate experiments, Dobreva et al. (2005) demonstrated that Elderly subjects are able to localize visual targets accurately with peripheral vision (SG = 1.02). This finding, in addition to normal visual acuity and fields demonstrated by all subjects in this study, suggests that visual perception in the Elderly does not play a significant role in the age-related differences observed here. As might be expected with aging, Elderly subjects demonstrated elevated auditory thresholds at higher frequencies (46.5 ± 27.2 and 72 ± 11.8 dB HL at 8 and 12 kHz, respectively). However, localization in Az is an ITD dominated process, and therefore unlikely to be affected by high-frequency hearing loss. Indeed, the ability of older subjects to localize auditory stimuli in Az appears to be unaffected by the spectrum of the target as long as it includes lower frequencies: localization accuracy and SG were shown to be comparable between broadband (0.1–20 kHz) and low-frequency (0.1–1 kHz) targets (Dobreva 2010).
Changes in eye position have been associated with changes in perception of “straight-ahead” along with co-registration of auditory space (Bohlander 1984; Lewald and Ehrenstein 2000; Weerts and Thurlow 1971), and we have previously shown that PSA shifts in the direction of eye position during eccentric fixation in young subjects (Cui et al. 2010). Interestingly, and in contrast to sound localization, the shift in PSA remained unaffected by aging. Therefore, the shift in PSA does not seem to be tied directly to sound localization per se, but instead appears to be a separate effect of ocular displacement that persists with advancing age. This is consistent with the concept of a “set-point” adapter that we proposed previously (Razavi et al. 2007). Such an adaptor responds slowly to a new spatial “zero”, defined by a new eye position. It does so by adjusting auditory and egocentric (body-centered) space in the direction of the new “zero”, but at different rates for different functions, and with potentially different final magnitudes of change. Such an adaptor might be utilized during development as a way of adjusting to the dynamic interaction between the environment and the maturing nervous system (including the oculomotor system), but following maturity has continued to exert its effect, perhaps for continued advantage in adjusting to ever present threats of disease and injury. Being an adaptive process, it is perhaps not surprising that its effects are attenuated in senescence.
Perhaps our results were influenced by the use of broadband auditory targets, which activate all potential spatial cues (and channels of processing), and might be differentially modified with age. Though we did not directly address this issue in the elderly, past studies indirectly examined this possibility (Cui et al. 2010). In those studies, young subjects localized randomly interleaved high-frequency (3–20 kHz), low-frequency (0.1–1 kHz), and broadband (0.1–20 kHz) auditory stimuli while fixating visual reference spots at Ctr, L and R20°, and 20° up and down from Ctr. For low-frequency targets in particular, and despite a reduced ability to accurately localize low-frequency targets in El due to an absence of required spectral cues, spatial shifts still occurred in both Az and El that were comparable to those obtained for high-frequency and broadband targets. Thus, adaptation of auditory spatial perception to eye position crosses all spatial channels, and functions independently of SG as well as other channel-dependent factors. Based upon these findings, we doubt that the high-frequency hearing loss characterizing peripheral presbycusis accounts for the age-related decline in the adaptive response of auditory space to eye position and suggest instead that a central etiology closely related to the adaptive circuitry itself is responsible for the effect. Experiments in which young subjects localize auditory targets with “old ears” simulating progressive high-frequency hearing loss are needed to test this hypothesis directly.
Acknowledgments
The authors thank Babak Razavi, Martin Gira, John Housel, and Scott H. Seidman for technical assistance. This work was supported by National Institutes of Health (NIH)-National Institute on Aging Grant R01-AG16319, NIH-National Institute on Deafness and Other Communication Disorders (NIDCD) Grant P30-DC05409 (Center for Navigation and Communication Sciences), and NIH-National Eye Institute Grant P30-EY01319 (Center for Visual Science). QC is a trainee in the Medical Scientist Training Program funded by NIH T32-GM07356, and was supported by a training grant from the NIH-NIDCD(F30-DC009372). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or NIH.
Contributor Information
Qi N. Cui, Department of Neurobiology and Anatomy, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Rochester, NY 14642-8603, USA
William E. O’Neill, Department of Neurobiology and Anatomy, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Rochester, NY 14642-8603, USA Center for Navigation and Communication Sciences, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Rochester, NY 14642-8603, USA.
Gary D. Paige, Department of Neurobiology and Anatomy, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Rochester, NY 14642-8603, USA Biomedical Engineering, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Rochester, NY 14642-8603, USA; Center for Navigation and Communication Sciences, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Rochester, NY 14642-8603, USA; Center for Visual Science, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Rochester, NY 14642-8603, USA.
References
- Abel SM, Hay VH. Sound localization. The interaction of aging, hearing loss and hearing protection. Scand Audiol. 1996;25:3–12. doi: 10.3109/01050399609047549. [DOI] [PubMed] [Google Scholar]
- Abel SM, Giguere C, Consoli A, Papsin BC. The effect of aging on horizontal plane sound localization. J Acoust Soc Am. 2000;108:743–752. doi: 10.1121/1.429607. [DOI] [PubMed] [Google Scholar]
- Alais D, Burr D. The ventriloquist effect results from near-optimal bimodal integration. Curr Biol. 2004;14:257–262. doi: 10.1016/j.cub.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Battaglia PW, Jacobs RA, Aslin RN. Bayesian integration of visual and auditory signals for spatial localization. J Opt Soc Am A Opt Image Sci Vis. 2003;20:1391–1397. doi: 10.1364/josaa.20.001391. [DOI] [PubMed] [Google Scholar]
- Bohlander RW. Eye position and visual attention influence perceived auditory direction. Percept Mot Skills. 1984;59:483–510. doi: 10.2466/pms.1984.59.2.483. [DOI] [PubMed] [Google Scholar]
- Butler RA, Humanski RA. Localization of sound in the vertical plane with and without high-frequency spectral cues. Percept Psychophys. 1992;51:182–186. doi: 10.3758/bf03212242. [DOI] [PubMed] [Google Scholar]
- Cui QN, Razavi B, O’Neill WE, Paige GD. Perception of auditory, visual, and egocentric spatial alignment adapts differently to changes in eye position. J Neurophysiol. 2010;103:1020–1035. doi: 10.1152/jn.00500.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobreva MS. Unpublished PhD thesis. University of Rochester; 2010. The influence of aging, memory, and stimulus characteristics on human auditory and visual spatial localization. [Google Scholar]
- Dobreva MS, O’Neill WE, Paige GD. Effect of memory and aging on auditory and visual spatial localization. Soc Neurosci Abstr. 2005;35:282–283. [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear Res. 1997;106:95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Gates GA, Cooper JC, Jr, Kannel WB, Miller NJ. Hearing in the elderly: the Framingham cohort, 1983–1985. Part I. Basic audiometric test results. Ear Hear. 1990;11:247–256. [PubMed] [Google Scholar]
- Getzmann S. The effect of eye position and background noise on vertical sound localization. Hear Res. 2002;169:130–139. doi: 10.1016/s0378-5955(02)00387-8. [DOI] [PubMed] [Google Scholar]
- Hebrank J, Wright D. Spectral cues used in the localization of sound sources on the median plane. J Acoust Soc Am. 1974;56:1829–1834. doi: 10.1121/1.1903520. [DOI] [PubMed] [Google Scholar]
- Lewald J. Eye-position effects in directional hearing. Behav Brain Res. 1997;87:35–48. doi: 10.1016/s0166-4328(96)02254-1. [DOI] [PubMed] [Google Scholar]
- Lewald J. The effect of gaze eccentricity on perceived sound direction and its relation to visual localization. Hear Res. 1998;115:206–216. doi: 10.1016/s0378-5955(97)00190-1. [DOI] [PubMed] [Google Scholar]
- Lewald J, Ehrenstein WH. The effect of eye position on auditory lateralization. Exp Brain Res. 1996;108:473–485. doi: 10.1007/BF00227270. [DOI] [PubMed] [Google Scholar]
- Lewald J, Ehrenstein WH. Auditory-visual spatial integration: a new psychophysical approach using laser pointing to acoustic targets. J Acoust Soc Am. 1998;104:1586–1597. doi: 10.1121/1.424371. [DOI] [PubMed] [Google Scholar]
- Lewald J, Ehrenstein WH. Visual and proprioceptive shifts in perceived egocentric direction induced by eye-position. Vision Res. 2000;40:539–547. doi: 10.1016/s0042-6989(99)00197-2. [DOI] [PubMed] [Google Scholar]
- Lewald J, Getzmann S. Horizontal and vertical effects of eye-position on sound localization. Hear Res. 2006;213:99–106. doi: 10.1016/j.heares.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Metzger RR, Mullette-Gillman OA, Underhill AM, Cohen YE, Groh JM. Auditory saccades from different eye positions in the monkey: implications for coordinate transformations. J Neuro-physiol. 2004;92:2622–2627. doi: 10.1152/jn.00326.2004. [DOI] [PubMed] [Google Scholar]
- Noble W, Byrne D, Lepage B. Effects on sound localization of configuration and type of hearing impairment. J Acoust Soc Am. 1994;95:992–1005. doi: 10.1121/1.408404. [DOI] [PubMed] [Google Scholar]
- Rakerd B, Vander Velde TJ, Hartmann WM. Sound localization in the median sagittal plane by listeners with presbyacusis. J Am Acad Audiol. 1998;9:466–479. [PubMed] [Google Scholar]
- Razavi B, O’Neill WE, Paige GD. Auditory spatial perception dynamically realigns with changing eye position. J Neurosci. 2007;27:10249–10258. doi: 10.1523/JNEUROSCI.0938-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH. Rapidly induced auditory plasticity: the ventriloquism aftereffect. Proceedings of the National Academy of Sciences USA. 1998;95:869–875. doi: 10.1073/pnas.95.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffler SK, Butler RA. Factors that influence the localization of sound in the vertical plane. J Acoust Soc Am. 1968;43:1255–1259. doi: 10.1121/1.1910976. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Further Observations on the Pathology of Presbycusis. Archives of Otolaryngology. 1964;80:369–382. doi: 10.1001/archotol.1964.00750040381003. [DOI] [PubMed] [Google Scholar]
- Weerts TC, Thurlow WR. The effects of eye position and expectation on sound localization. Percept Psychophys. 1971;9:35–39. [Google Scholar]
- Yao L, Peck CK. Saccadic eye movements to visual and auditory targets. Exp Brain Res. 1997;115:25–34. doi: 10.1007/pl00005682. [DOI] [PubMed] [Google Scholar]


