Abstract
Histotripsy is a tissue ablation method that utilizes focused high amplitude ultrasound to generate a cavitation bubble cloud that mechanically fractionates tissue. Effective histotripsy depends on initiation, control, and maintenance of cavitation bubble clouds in the targeted area. In this study, we hypothesized that a low pressure acoustic pulse sequence applied before and/or during histotripsy therapy would increase the cavitation initiation pressure threshold and growth of cavitation bubble clouds. This technique could shrink or “sharpen” the focal zone during histotripsy to produce more precise and well defined lesions with minimal collateral damage. It may also be a way to actively protect soft tissue from cavitation damage during lithotripsy by increasing the pressure threshold for bubble cloud initiation. We applied these low amplitude acoustic pulse sequences before and during histotripsy treatments with PRF of 1 and 100 Hz, in three different mediums: water, tissue phantom agarose gel, and bovine liver in-vitro. Acoustic backscatter signals and optical imaging were used to detect and monitor initiation, maintenance and growth of the resulting cavitation bubble cloud. Results demonstrated that the use of low amplitude acoustic pulse sequences could increase the cavitation pressure amplitude threshold by 20% in the targeted area.
Index Terms—: Histotripsy, Shock Scattering, lithotripsy, Cavitation Threshold, Bubble Coalescence, Cavitation Suppression, Pre-conditioning
I. INTRODUCTION
Histotripsy is a cavitation based ultrasound therapy that produces tissue fractionation through a mechanical process using controlled cavitation bubble clouds [1]-[5]. During histotripsy, a dense cloud of micron size cavitation bubbles is generated in a targeted area by applying a focused, microseconds length, high amplitude ultrasound burst at a very low duty cycle (typically less than 1%). Studies suggest the erosion caused by histotripsy is not achieved by liquefying the tissue through high temperature, but rather through mechanical stress caused by oscillation and collapse of cavitation bubble clouds [4],[5]. A histotripsy cavitation bubble cloud can be directly generated by applying a negative pressure exceeding a distinct threshold, which appears to be intrinsic to the medium of about 25 to 30 MPa in soft tissue [6]-[11]. Another way to initiate a dense bubble cloud is via the shock-scattering mechanism where incoming acoustic waves are reflected and inverted by pre-existing single bubbles at the focus. When the reflected waves combine with subsequent incoming cycles (particularly if there are highly shocked nonlinear waveforms due to nonlinear propagation), a sufficiently large negative pressure can be created to exceed the intrinsic threshold resulting in growth of a similarly dense bubble cloud [6],[7].
The primary motivation for this study is to develop a technique to reduce unintended tissue damage from cavitation during histotripsy or lithotripsy treatments by modulating the cavitation initiation pressure threshold. Specifically, this study investigates the incident pressure threshold to initiate and maintain bubble clouds through the shock-scattering mechanism when using various low amplitude acoustic pulse sequences. For histotripsy, effective therapy is highly dependent on generating and maintaining a dense bubble cloud at the targeted area [7]-[9]. Wang found that applying “cavitation suppressing” pulses to the periphery of the focus immediately before histotripsy pulses (called “preconditioning”) reduced bubble formation in the periphery of the focus, resulting in smaller and better confined lesions [12].
Cavitation initiation for shock-scattering displays a stochastic nature affected by existing bubble nuclei populations in the medium. We hypothesized that by applying proper low pressure amplitude pulse sequences before or during histotripsy therapy, the initiation pressure threshold and growth pattern of cavitation bubble clouds could be modified. In previous in-vivo and in-vitro studies, applying low amplitude acoustic pulse sequences has shown to increase the efficacy of therapy in histotripsy [13]-[16] and lithotripsy [17],[18] by minimizing the shielding effect of bubbles. The residual micron sized bubbles following a cavitation cloud collapse [19],[20] have long dissolution times on the order of 1 second and thus a large fraction persist between subsequent pulses at PRFs > 1 Hz [10],[21]-[26]. For subsequent pulses, these nuclei absorb energy from the negative pressure phase of the histotripsy waveform [25]. Previous studies suggest that low amplitude acoustic pulse sequences can overcome the adverse effects of persisting bubbles by forcing cavitation bubbles to coalesce, or disperse away from propagation path before the arrival of next therapy pulse [13],[17]. The same process of bubble coalescence may also have an effect on changing the initiation pressure threshold in shock-scattering histotripsy. In this study, we investigated the effect of applying low amplitude acoustic pulse sequences before and during shock scattering histotripsy on the cavitation initiation pressure threshold. We expect that the cavitation initiation pressure threshold for shock-scattering would increase due to a reduction in the probability of initial nuclei presence at the focus.
II. Methods
A. Experimental Setup
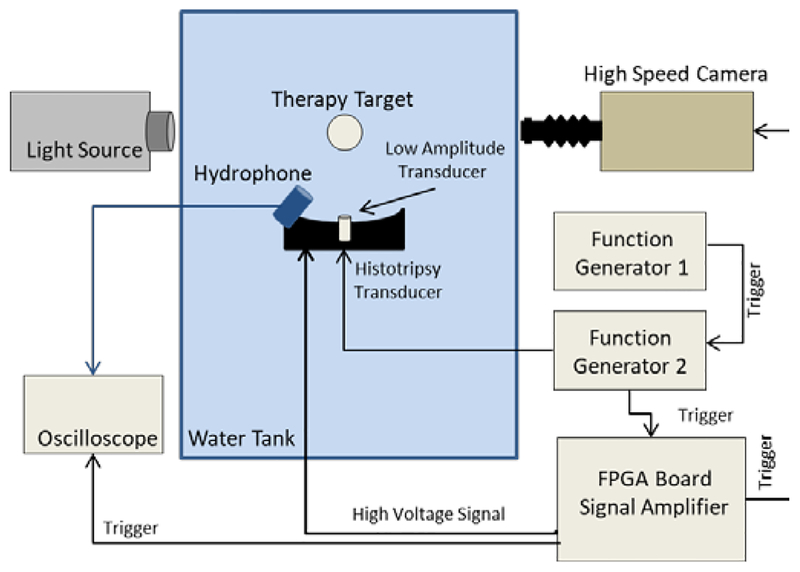
In a series of three experiments, histotripsy and low amplitude acoustic pulse sequences were applied targeting (I) a chamber of degassed water, (II) tissue phantom agarose gel, and (III) bovine liver tissue samples. Acoustic backscatter signals and optical imaging were used to detect and monitor initiation, maintenance and growth of resulting cavitation bubble cloud (Fig. 1).
Fig. 1.
Schematic of the experimental setup. Histotripsy and low amplitude acoustic pulse sequences were applied targeting (I) a chamber of degassed water, (II) tissue phantom agarose gel, and (III) bovine liver tissue samples. Cavitation was monitored using acoustic backscatter signals and optical imaging obtained from a high speed camera positioned perpendicular to histotripsy transducer propagation axis.
A.I. Water
The transducers (described in sections B and C) were placed in a water tank filled with degassed water to a dissolved oxygen level of 80% of saturation, at room temperature measure by a Traceable Digital Oxygen Meter (Control Co., Friendswood, TX, USA). Histotripsy pulses targeted a chamber of degassed deionized water with saturation below 80%. The gas concentration in the chamber was kept constant for all experiments by a circulation pump. The chamber components were made from polytetrafluoroethylene, glass, and 316 stainless steel. It is 150 mL cavitation chamber and has two glass windows to facilitate high speed photography, and two acoustic windows made from 12 μm-thick low density polyethylene membranes [8]. 4–6 trials were performed for each of the parameters.
A.II. Tissue Phantom Agarose Gel
Tissue phantoms were prepared by mixing agarose powder (Agarose Type VII, Sigma-Aldrich) with deionized water at 1% ratio. The solution was stirred and heated in a microwave until completely dissolved, degassed under high vacuum for half an hour, and then poured in a cylindrical gel holder 6 cm diameter and 7 cm height. It was then put into a refrigerator for an hour in order for the gel to solidify. After preparation, the phantom gel holder was mounted to an electronically controlled positioning system (VXM Stepping Motor Controller, Velmex Inc., Bloomfield, NY) in the water tank filled with degassed water with dissolved oxygen level of 15%-20% of saturation. Horizontal and vertical spacing of 7 mm was used between treatment spots for all of the trials of each set of parameters to minimize possible variations of the acoustic path and influence of previous treatments. The results of 10 trials for each of the parameters were collected.
A.III. Bovine Liver Tissue
Cavitation threshold modulation experiments were repeated on bovine liver samples in-vitro for a subset of parameters. Whole livers were harvested and placed in degassed saline immediately after slaughter for transport. At the laboratory, these were sectioned into 5 cm cubes avoiding large vessels and then degassed under vacuum in saline for 6 hours. The pieces were then placed in a holder surrounded by 1% agarose (Agarose, Low Melt. Scientific Inc. Burton, MI) gel prepared the same as in A.II. After one hour cooling, the solidified gel with embedded tissue samples were ready for experiments. Samples were mounted on the positioning system and moved in steps of 5 mm horizontally and vertical in one central slice for each of the treatment spots. Water in the tank was highly degassed to below 15%. Since cavitation events cannot be observed by optical imaging in tissue, acoustic backscatter signals were the only measure for cavitation detection in the tissue experiments. 8–10 trials for each of the parameters were performed for histotripsy PRF of 100 Hz.
B. Acoustic Pulse Sequence
Histotripsy pulses were generated by a 1 MHz focused piezocomposite ultrasound transducer with 10 cm aperture and 9 cm focal length, with a hole in the center (Imasonic, Voray sur l’Ognon, France). The histotripsy transducer was driven by an in-house made system at a range of different pressure levels with negative peak/positive peak between 8/8 and 28/50 MPa. Measurements and calibration of histotripsy waveform pressure was done by an in-house built fiber optic probe hydrophone [27] in degassed water at room temperature for lower pressures and extrapolated for higher pressures; since measurements for pressures higher than the intrinsic pressure cannot be done accurately due to cavitation initiation at the tip of the fiber. All calibrations were performed in degassed water. Pressure levels that are reported for experiments with agarose gel and liver tissue as targets in water tank are based on calibrated pressures in degassed water as well.
Low amplitude acoustic pulse sequences were produced by an in-house made single element transducer constructed from a 1 MHz disc of PZ36 material (Ferroperm Piezoceramics A/S, Kvistgaard, Denmark), with 20mm diameter and 1.5 mm thickness with an Accura 60 (3D Systems Inc., Rock Hill, SC, USA) acoustic lens matching layer. This transducer was driven by an ENI power amplifier (ENI, Inc., Rochester, NY) model AP 400B controllable power amplifier, for experiments in water and in agarose gel, and model A150 RF power amplifier for experiments in liver tissue. This transducer was placed in the central hole of the histotripsy transducer. The near field distance of the low amplitude transducer is about 67.5 mm, however, since the −3 dB beamwidth is about 85.0 mm in axial direction and 6.2 mm in lateral, this sound field fully covers the focus zone of histotripsy transducer and the extent of growth of the bubble cloud (less than 3 mm in lateral and 10 mm in axial direction in agarose gel), which grows toward the transducer along the axial axis [7]. The low amplitude transducer was calibrated by an HNR-0500 needle hydrophone (Onda Corporation, Sunnyvale, CA) for experiments in water and agarose gel, and an identical second transducer was used in experiments in liver tissue that was calibrated by a HGL hydrophone (hydrophone: ONDA HGL 0085, and amplifier: ONDA AH2010. ONDA Sunnyvale, Ca).
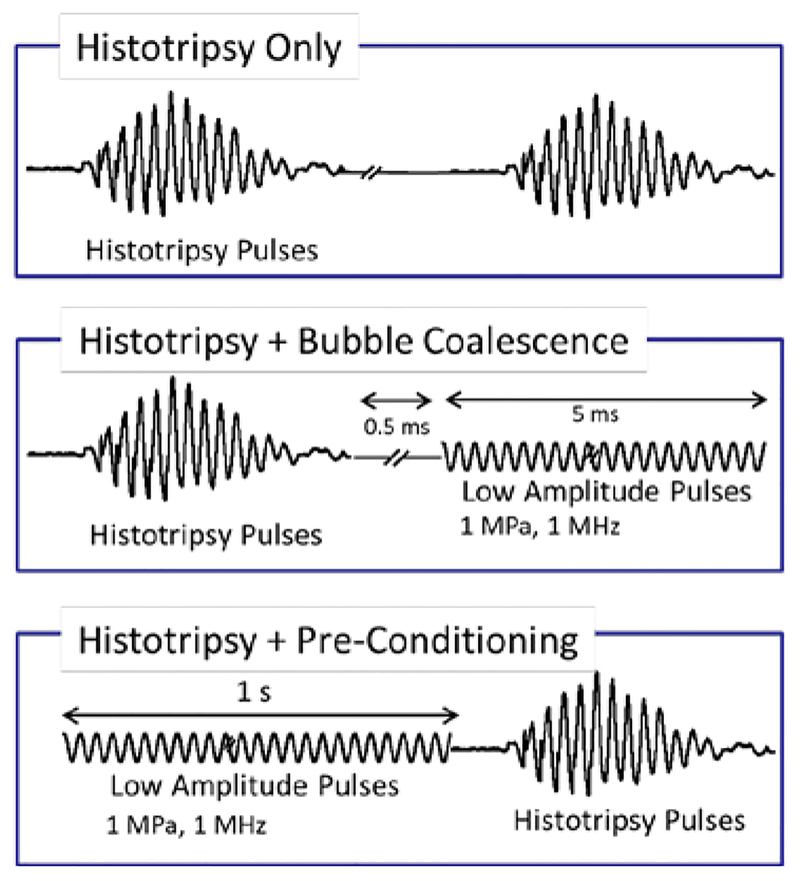
The experiments were performed with three different pulse schemes as shown in fig. 2. First case was histotripsy only, in which 5 cycles of 1 MHz histotripsy pulses were fired at PRF 1, 10, or 100 Hz, with peak negative pressures from 10 to 28 MPa for 100 pulses. In the second case, bubble coalescence, each histotripsy pulse was followed by burst of 5,000 cycles of low amplitude acoustic pulses with pressure amplitude of 1 MPa, which were fired 500 μs (100 μs for experiments in liver tissue) after histotripsy pulses in order to allow the histotripsy cavitation bubble cloud to grow and collapse without interference. For the third case, preconditioning, a full second of low amplitude acoustic pulses with same pressure amplitude were applied before histotripsy treatment pulses.
Fig. 2.
Three different pulse schemes for threshold modulation studies. Case 1: Histotripsy Only, in which five cycles of 1 MHz histotripsy pulses were applied for a range of different pressures. Case2: Histotripsy+Bubble Coalescence, in which each histotripsy pulse is followed by low amplitude acoustic pulses for duration of 5 ms. Case 3: Histotripsy+Pre-Conditioning, in which one full second of low amplitude acoustic pulses were applied immediately before the start of histotrispy treatment.
C. Detection and Monitoring
In these experiments, we used two different methods for monitoring and detecting cavitation events; the amplitude of the backscattered signal and the back-lit bubble cloud size.
The main measure for cavitation detection was the backscattered signal that was captured by an uncalibrated low frequency unfocused marine hydrophone (H1a, Aquarian Audio Products, Anacortes, WA), facing the focus of therapy transducer at about 30° angle; an increase in amplitude of the backscattered signal is an indicator for bubble cloud initiation. The energy of the acoustic backscatter signal of each pulse was calculated as the sum of voltage squared samples of each pulse, and normalized to the spatial peak pulse average intensity of the corresponding therapy pulses. The threshold for cavitation detection was then defined as the average of the uninitiated acoustic backscatter energy plus three standard deviations.
For experiment in water, the optical images from a high speed 1 megapixel CCD camera (Phantom V210, Vision Research) were recorded, and the area of backlit bubble cloud in each frame was checked against a threshold. The camera was triggered after each histotripsy pulse to record one image per pulse after about 70 μs delay to account for the sound traveling time from histotripsy transducer to the focus as well as the growth of bubble cloud. Bubble cloud was backlit by a continuous light source. For experiments in agarose gel a digital, 1.3-megapixel, CMOS camera (PN: FL3-U3-13Y3M-C, Flea 3, PointGrey, Richmond, BC, Canada) was used in the same setting, and for experiment in bovine liver tissue, no optical images were obtained.
In this study, bubble cloud initiation pressure threshold was defined as the lowest pressure at which bubble cloud is detected with probability of at least 50%, or the pressure at which the sigmoid curve fits to the data (in fig. 3,5 and 6) given the probability=0.5 when pressures exceeding the 50 percentile is not available. The percentage of pulses in which bubble cloud was detected is another measure used to evaluate the results.
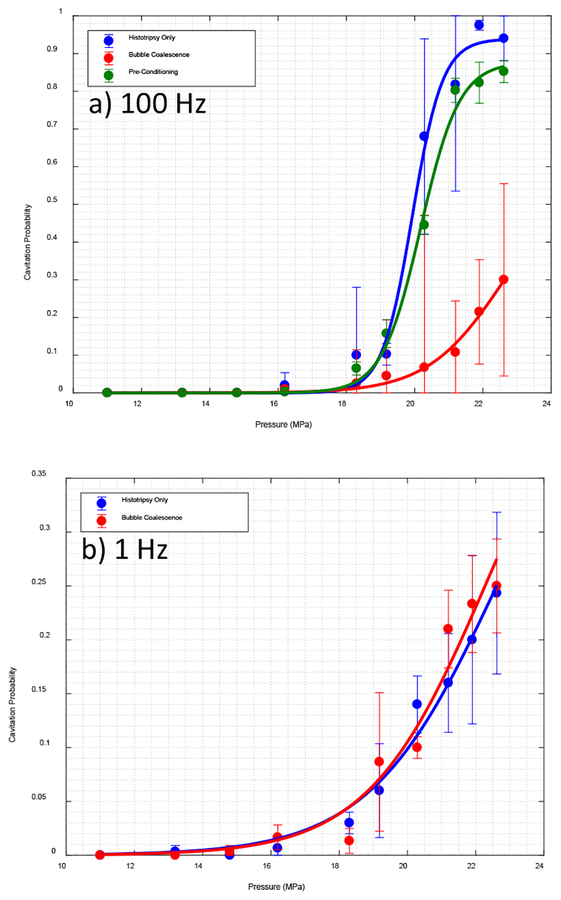
Fig. 3.
Cavitation probability for experiments in water for a) PRF 100 Hz and b) PRF 1 Hz
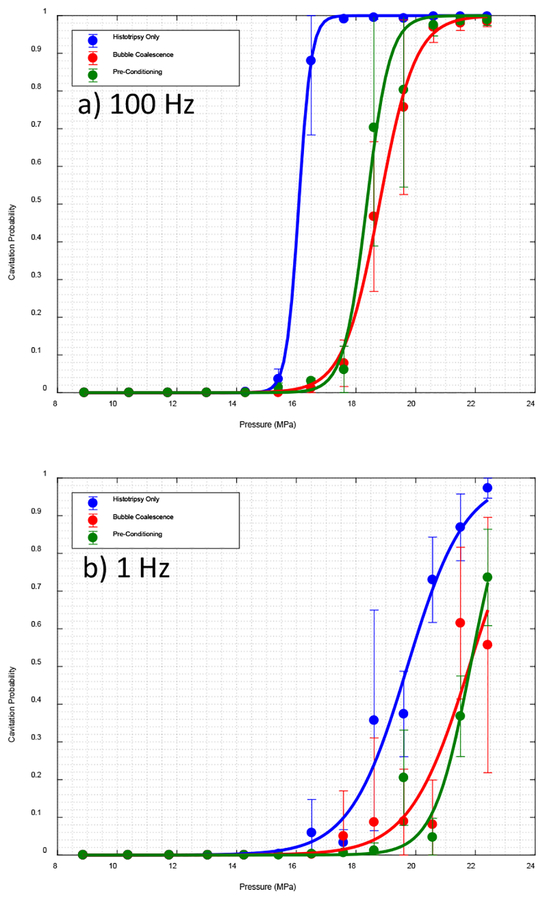
Fig. 5.
Cavitation probability for experiments in tissue phantom agarose gel for a) PRF 100 Hz and b) PRF 1 Hz
Fig. 6.
Cavitation probability for treatments in liver tissue for PRF 100 Hz
III. Results
A. Experiment in Water
Fig. 3 shows the percentage of pulses for which a bubble cloud was detected for a) PRF of 100 Hz and b) 1 Hz. All three cases were completed for PRF of 100 Hz, the results for pre-conditioning were not as significant even for 100 Hz, and as a result we did not repeat pre-conditioning case for 1 Hz. For PRF of 100 Hz, applying low amplitude acoustic pulse sequences during histotripsy significantly reduced the probability of cavitation initiation, effectively increasing the cavitation initiation pressure threshold. The threshold was increased from 20.3 MPa for histotripsy only chase to 21.2 MPa for pre-conditioning case, and to 24.5 MPa for bubble coalescence case. By Kolmogorov–Smirnov test over the non-zero part of the curves with 95% confidence level we can conclude that the three fit curves are statistically from different distributions. Kolmogorov–Smirnov test shows asymptotic p-value = 2.19e-4 between histotripsy only and bubble coalescence, and p = 0.047 for histotripsy only and pre-conditioning. At 1 Hz, no statistically significant difference was observed between histotripsy only chase and bubble coalescence case (p-value=0.41) and the predicted pressure threshold that fit the curve in fig. 3b is 32.1 MPa for both cases. The asymptotic p-value = 0.19 per Kolmogorov–Smirnov test between the two curves in figure 3 b shows no statistically significant difference.
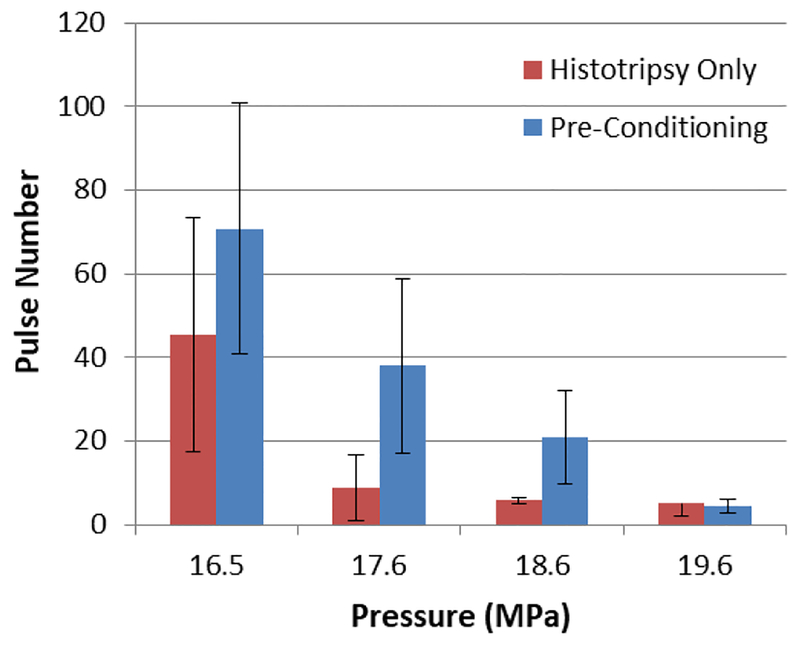
Investigating the backscattered signal, we realized that the effect of pre-conditioning at PRF of 100Hz is only significant in the beginning of the treatment. In other words applying pre-conditioning is delaying the cavitation initiation, and after this delay of up to 30 pulses the same pattern is observed; fig. 4 shows the pulse number at which bubble cloud initiation occurs for pressures close to threshold. In each of the first three pressures initiation is delayed when pre-conditioning is applied.
Fig. 4.
Number of pulses required to initiate cavitation bubble cloud for experiments in water for histotripsy only case and pre-conditioning with PRF 100 Hz.(p-value = 0.04)
B. Experiment in Tissue Phantom Agarose Gel
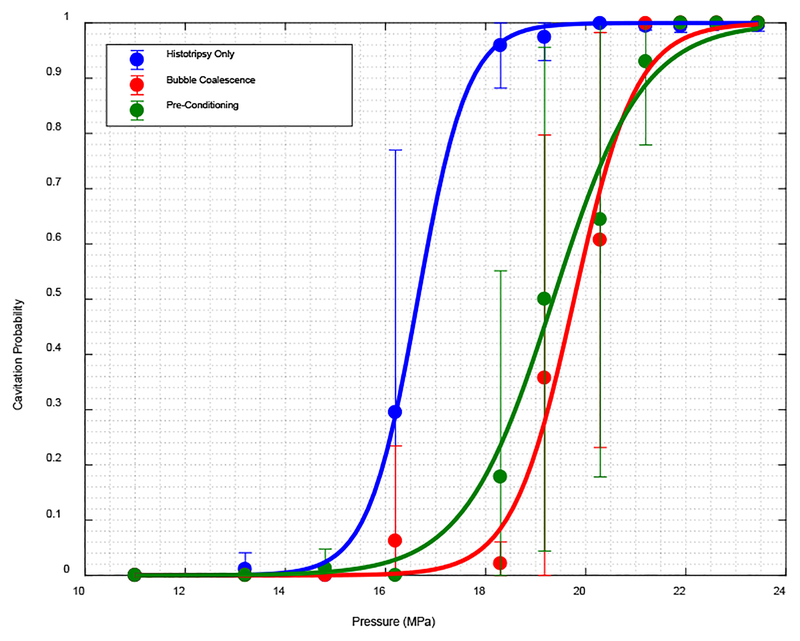
Results from experiments in agarose gel demonstrated that the applying low amplitude acoustic pulse sequences can increase the cavitation threshold in the targeted area. Fig. 5 show the cavitation probability for different pressures at a) PRF of 100 and b) 1 Hz.
For PRF of 100 Hz, cavitation initiation threshold for both cases of bubble coalescence and pre-conditioning was increased from 16.5 MPa to 19.6 MPa. We observed a statistically significant difference between histotripsy only case and both bubble coalescence and pre-conditioning cases. Based on Kolmogorov–Smirnov test on non-zero part of the curves we can assert that the data points are from different distributions with asymptotic p-value of 3.11e-11 for histotripsy only and bubble coalescence curve, and 1.28e-7 between histotripsy only case and pre-conditioning curves.
For PRF of 1 Hz, cavitation initiation threshold for both cases of bubble coalescence and pre-conditioning was increased from 18.6 MPa to 21.5 MPa for 1 Hz PRF. Similarly, a statistically significant difference between histotripsy only case and both bubble coalescence and pre-conditioning cases was observed. Based on Kolmogorov–Smirnov test on non-zero part of the curves we can assert that the data points are from different distributions with asymptotic p-value of 0.024 for histotripsy only and bubble coalescence curve, and 1.47e-9 between histotripsy only case and pre-conditioning curve.
Unlike open water, the increase in threshold was maintained in lower PRF as well.
C. Experiment in Bovine Liver Tissue
The results from the experiment in tissue showed similar results to those obtained from experiments in the agarose tissue phantom gel. Applying low amplitude acoustic pulse sequences in both bubble coalescence and pre-conditioning cases resulted in an increase in the cavitation initiation threshold; for a PRF of 100 Hz, the threshold increased from 18 MPa for histotripsy only to about 20.3 MPa for both bubble coalescence and pre-conditioning cases. We observed statistically significant differences between all three cases. Based on Kolmogorov–Smirnov test on non-zero part of the curves we can assert that the data points are from different distributions with asymptotic p-value of 3.37e-5 for histotripsy only and bubble coalescence curve, and 1.65e-6 between histotripsy only case and pre-conditioning curves.
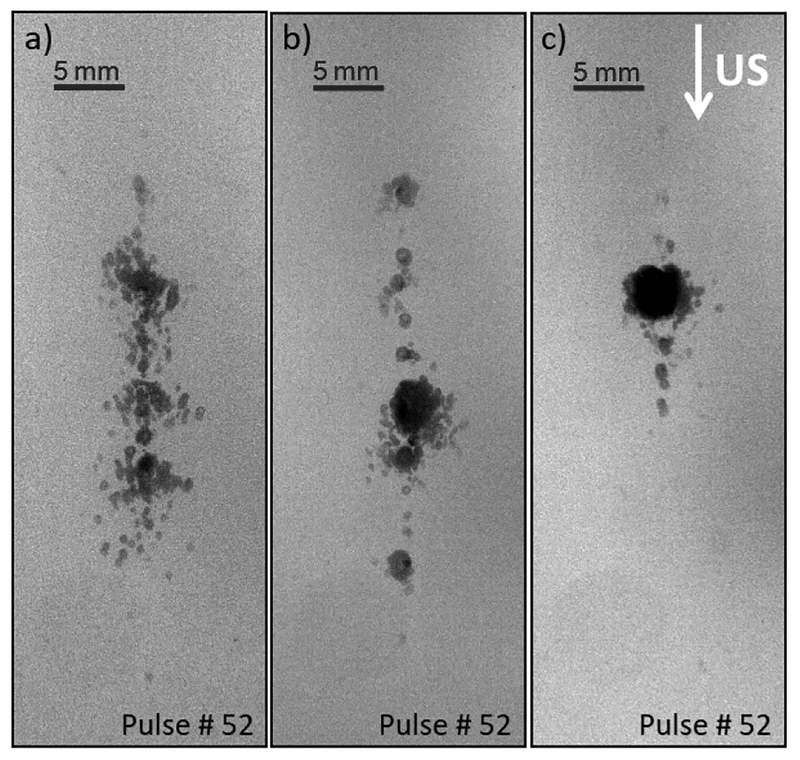
Qualitative analysis of backlit bubble cloud images showed that the shape and density of the resulting bubble cloud can also be modified through utilizing low amplitude acoustic pulses before and during treatment. By applying the low amplitude acoustic pulses we were able to generate a dense cavitation bubble cloud at the focus, which would increase efficacy of the treatment, while decreasing scattered cavitation in the peripheral zone, which would reduce collateral damage. The size and shape differences of the resulting bubble cloud by shock scattering when pre-conditioning and bubble coalescence pulses were applied are illustrated in fig. 7.
Fig. 7.
Cavitation bubble cloud in agarose gel generated by shock scattering at 22MPa with PRF 100Hz, a) histotripsy only, b) histotripsy with pre-conditioning, and c) histotripsy with bubble coalescence pulses.
IV. Discussion
The results of this study suggest it may be possible to protect tissue during histotripsy or lithotripsy treatments by applying low amplitude acoustic pulse sequences to modulate the cavitation threshold. This mechanism could be used for tissue hardening, active focal sharpening, and protecting sensitive tissues or structures located close to a treatment target. Results from experiments in all three mediums of water, tissue phantom gel, and liver tissue generally confirmed our hypothesis and showed that applying low amplitude acoustic pulse sequences before or during shock scattering histotripsy treatment increases the cavitation initiation pressure threshold. For experiments in water at the low PRF of 1 Hz, we did not observe any statistically significant difference when low amplitude acoustic pulse sequences were applied for pre-conditioning or bubble coalescence cases. At higher rate, PRF of 100 Hz, the effect of low amplitude acoustic pulse sequences applied after each histotripsy pulse, the bubble coalescence case, was much more significant than pre-conditioning; pre-conditioning effect was only significant in the beginning of the treatment, after which generation and shape of the bubble cloud was similar to those of the histotripsy only cases. However, in experiments in agarose tissue phantom gel and in liver tissue, the effect of pre-conditioning persisted throughout the treatment and the results were similar to bubble coalescence cases. The difference in persistence of effect of pre-conditioning in water in comparison with agarose gel and tissue was expected since, in free water due to streaming, distribution of cavitation nuclei can change more rapidly than in agarose gel and soft tissue. Despite the differences between pre-conditioning and bubble coalescence cases; pre-conditioning reduces pre-existing cavitation, and coalescence reduces residual cavitation by forced coalescence, still some of the same physical mechanism of forced coalescence is assumed to be in play in pre-conditioning as well. In pre-conditioning, similar to coalescence case, reduction of pre-existing nuclei at the focus and periphery of the focus by forced coalescence and dispersion, results in less scattered cavitation and a denser cavitation bubble cloud. Although the bubble clouds in pre-conditioning case and bubble coalescence case shown in figure 7 are not exactly similar, both has less scattered cavitation in comparison with histotripsy only case.
Cavitation initiation, particularly in shock scattering histotripsy, displays a stochastic nature affected by existing nuclei populations in the medium. By applying low amplitude acoustic pulse sequences during treatment, the residual nuclei are forced to coalesce. As a result of active bubble coalescence, the number existing bubbles in the vicinity of focus decreases, which consequently, reduces the probability of nuclei presence at the focus when the next therapy pulse arrives. Shock scattering from some existing nuclei at the focus is to generate a high negative pressure and initiate histotripsy bubble cloud. As a result, probability of shock scattering at any pressure level close to threshold is decreased, which translates to increase of the pressure threshold.
It is helpful to note that low amplitude acoustic pulse sequences or cavitation suppression pulses, as previously demonstrated in a number of studies [13]-[18], can be utilized to increase the efficacy of histotripsy and lithotripsy treatments at higher PRFs by avoiding or minimizing the memory effect and shielding effect of residual cavitation nuclei at the focal area and along the propagation path. At lower PRF, after each cavitation event there is sufficient time for a majority of the residual bubbles to passively dissolve away, while at higher rates the pulse efficacy is significantly reduced due to persisting bubbles. Memory effect mainly explains how residual bubbles at the focal area contribute to this reduced efficacy, and shielding effect explains the attenuation of histotripsy pulses at the focus. On subsequent pulses, pre-focal residual bubbles absorb energy from the negative pressure phase of the histotripsy waveform and distort the waveform and attenuate its amplitude. Cavitation suppression pulses can be utilized to stimulate residual cavitation bubbles to coalesce and clear the path for the subsequent histotripsy pulses. Since cavitation suppression pulses in the current experimental setup design would result in removing bubbles from the propagation path as well as focal area, it would result in reducing attenuation of therapy signal at higher rates. This process potentially contributes to initiating and maintaining histotripsy bubble cloud at lower pressures. However, we still observed significant increase in initiation threshold when cavitation suppression pulses were used despite this process working in opposite direction. Therefore, we would expect to observe even more pronounced increase in initiation pressure threshold, with different experiment setup with cavitation suppression transducer firing at the focus perpendicular to the propagation path of histotripsy transducer. Consequently, if the same experiments were performed with histotripsy at intrinsic threshold instead of histotripsy by means of shock scattering, we would expect to observe decrease in initiation pressure threshold.
In this study, low pressure acoustic pulses were utilized during treatment to stimulate residual cavitation bubbles to coalesce. This mechanism occurs through the acoustic field, inducing size oscillations in the bubbles, and gives rise to two major forces [28]-[35]. The primary Bjerknes force describes the tendency of bubbles smaller than resonant size to move up a pressure gradient and congregate at pressure antinodes, and bubbles larger than resonant size to move down a pressure gradient and congregate at pressure nodes. The secondary Bjerknes force describes an attractive force between bubbles which are oscillating in phase with one another, and a repulsive force between bubbles which are oscillating out of phase with one another, depending on their size and the acoustic field frequency. This secondary Bjerknes force is hypothesized to be the dominant factor bringing the remnant bubbles together to coalesce. During stable bubble oscillations, this force increases with higher amplitude and higher frequency of the driving sound field. The amplitude and frequency of cavitation suppression pulses in this study are set to mechanical index of one which would result in stable oscillation and maximum bubble coalescence [15]. Frequency of both pre-conditioning and bubble coalescence pulses were the same in these preliminary studies, each of which is subject to further optimization. The efficacy of forced bubble coalescence for a given bubble population is highly dependent on the driving frequency, particularly if its corresponding resonant size is close to the existing bubble nuclei size range. Based on the Minnaert formula [36], resonant size of driving frequency in this study is about diameter of 6 μm, which is in the range of micron size cavitation nuclei following the collapse of histotripsy bubble cloud, while range of stable and incidental bubbles prior to treatment is assumed to be three orders of magnitude smaller in range nanometer-sized nuclei [8,37–39]. Furthermore, pre-conditioning process is expected to rely on dispersion (primary Bjerknes forces), as well as coalescence (secondary Bjerknes forces), and as a result, since there are different phenomena in play in each mechanism, separate optimization for each mechanism, or combination of the two, can lead to even more pronounced change in initiation pressure threshold.
Acknowledgment
Funding for this study was provided by a grant from the National Institutes of Health R01 DK091267. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biographies

Hedieh Alavi Tamaddoni is currently a PhD student in Biomedical Engineering at University of Michigan, Ann Arbor, MI. She received her M.S.E. degree in Electrical Engineering from University of Michigan in 2013, and her B.S. degree in Electrical Engineering from Virginia Tech, Blacksburg, VA. Her fields of research are signal and image processing, histotripsy and therapeutic ultrasound.

Alexander P. Duryea received the B.S.E., M.S.E., and Ph.D. degrees from the University of Michigan, Ann Arbor, MI, USA, in 2009, 2010, and 2015, respectively, all in biomedical engineering. He is currently a Senior Ultrasound Engineer with HistoSonics, Inc., Ann Arbor. His dissertation research focused on non-invasive ultrasound therapy, with an emphasis on the application of histotripsy to the treatment of renal calculi. He is continuing work on the development of histotripsy for various indications with HistoSonics.

Eli Vlaisavljevich received the B.S. degree from Michigan Technological University, Houghton, MI, USA, in 2010, and the M.S. and Ph.D. degrees from the University of Michigan, Ann Arbor, MI, USA, in 2013 and 2015, respectively, all in biomedical engineering. He is currently a Postdoctoral Fellow with the Department of Biomedical Engineering, University of Michigan. His current research interests include ultrasound for noninvasive tissue modulation and ablation, acoustic cavitation, cancer, tissue engineering, and tissue mechanics. Dr. Vlaisavljevich received selected honors, includes a Goldwater Scholarship, a Michigan Tech Provost’s Award for Scholarship, an NSF Graduate Research Fellowship, and a Towner Prize for Outstanding Ph.D. Research.

Zhen Xu (S’05–M’06) received the B.S.E. (highest Hons) degree in biomedical engineering from Southeast University, Nanjing, China, in 2001, and the M.S. and Ph.D. degrees in biomedical engineering from the University of Michigan, Ann Arbor, MI, USA, in 2003 and 2005, respectively. She is currently an Associate Professor with the Department of Biomedical Engineering, University of Michigan. Her research interests include ultrasound therapy, particularly the applications of histotripsy for noninvasive surgeries. Dr. Xu received the Ultrasonics, Ferroelectrics, and Frequency Control (UFFC) Outstanding Paper Award in 2006; the American Heart Association Outstanding Research in Pediatric Cardiology in 2010; the National Institute of Health New Investigator Award at the First National Institute of Biomedical Imaging and Bioengineering Edward C. Nagy New Investigator Symposium in 2011; and the Frederic Lizzi Early Career Award from ISTU in 2015. She is currently an Associated Editor of the IEEE Transactions on Ultrasound, Ferroelectrics, and Frequency Control, the Women in Engineering Chair of UFFC, and a Board Member of the International Society of Therapeutic Ultrasound.

Timothy L. Hall was born in Lansing, MI, USA, in 1975. He received the B.S.E. and M.S.E. degrees in electrical engineering and the Ph.D. degree in biomedical engineering from the University of Michigan, Ann Arbor, MI, USA, in 1998, 2001, and 2007, respectively. He was with Teradyne Inc., Boston, MA, USA, as a Circuit Design Engineer from 1998 to 1999, and also with the University of Michigan, as a Visiting Research Investigator from 2001 to 2004, where he is currently an Assistant Research Scientist with the Department of Biomedical Engineering. His research interests include high-power pulsed-RF-amplifier electronics, phased-array ultrasound transducers for therapeutics, and sonic cavitation for therapeutic applications.
Footnotes
Disclosure
A. P. Duryea, E. Vlaisavljevich, Z. Xu and T.L. Hall have financial interests and/or other relationships with HistoSonics Inc.
References
- [1].Xu Z, Ludomirsky A, Eun LY, Hall TL, Tran BC, Fowlkes JB, et al. , “Controlled ultrasound tissue erosion,” IEEE Trans Ultrason Ferroelectr Freq Control, vol. 51, no. 6, pp. 726–36, June 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Roberts WW, Hall TL, Ives K, Wolf JS Jr., Fowlkes JB, and Cain CA, “Pulsed cavitational ultrasound: a noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney,” J. of Urology, vol. 175, no. 2 pp. 734–8, February 2006. [DOI] [PubMed] [Google Scholar]
- [3].Parsons JE, Cain CA, Abrams GD, and Fowlkes JB, “Pulsed cavitational ultrasound therapy for controlled tissue homogenization,” Ultrasound in Medicine & Biology, vol. 32, no. 1, pp. 115–29, January 2006. [DOI] [PubMed] [Google Scholar]
- [4].Parsons JE, Cain CA and Fowlkes JB, “Spatial variability in acoustic backscatter as an indicator of tissue homogenate production in pulsed cavitational ultrasound therapy,” in IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, vol. 54, no. 3, pp. 576–590, March 2007. [DOI] [PubMed] [Google Scholar]
- [5].Xu Z, Fowlkes JB, Rothman ED, Levin AM, and Cain CA, “Controlled ultrasound tissue erosion: The role of dynamic interaction between insonation and microbubble activity,” Journal of the Acoustical Society of America, vol. 117, no. 1, pp. 424–435, January 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maxwell AD, Cain CA, Fowlkes JB and Xu Z, “Inception of cavitation clouds by scattered shockwaves,” 2010 IEEE International Ultrasonics Symposium, San Diego, CA, 2010, pp. 108–111. [Google Scholar]
- [7].Maxwell AD, Wang TY, Cain CA, Fowlkes JB, Sapozhnikov OA, Bailey MR, and Xu Z, “Cavitation clouds created by shock scattering from bubbles during histotripsy,” Journal of the Acoustical Society of America, vol. 130, pp. 1888–1898, October 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Maxwell AD, Cain CA, Hall TL, Fowlkes JB, and Xu Z, “Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials,” Ultrasound in Medicine & Biology, vol. 39, pp. 449–465, March 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vlaisavljevich E, Maxwell AD, Warnez M, Johnsen E, Cain CA, Xu Z, “Histotripsy-induced cavitation cloud initiation thresholds in tissues of different mechanical properties.” Ultrasonics, Ferroelectrics, and Frequency Control, IEEE Transactions, vol. 61, no.2, pp. 341–352. February 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fowlkes JB and Crum LA, “Cavitation threshold measurements for microsecond length pulses of ultrasound,” Journal of the Acoustical Society of America, vol. 83, no. 6, pp. 2190–2201, June 1988. [DOI] [PubMed] [Google Scholar]
- [11].Fowlkes JB, Carson PL, Chiang EH, and Rubin JM, “Acoustic generation of bubbles in excised canine urinary bladders,” Journal of the Acoustical Society of America, vol. 89, no. 6, pp. 2740–2744, June 1991e [DOI] [PubMed] [Google Scholar]
- [12].Wang TY, Xu Z, Hall TL, Fowlkes JB, Roberts WW and Cain CA, “Active focal zone sharpening for high-precision treatment using histotripsy,” IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, vol. 58, no. 2, pp. 305–315, February 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Duryea AP, Cain CA, Tamaddoni HA, Roberts WW and Hall TL, “Removal of residual nuclei following a cavitation event using low-amplitude ultrasound,” IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, vol. 61, no. 10, pp. 1619–1626, October 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Duryea AP, Roberts WW, Cain CA and Hall TL, “Removal of residual cavitation nuclei to enhance histotripsy erosion of model urinary stones,” IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, vol. 62, no. 5, pp. 896–904, May 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Duryea AP, Tamaddoni HA, Cain CA, Roberts WW and Hall TL, “Removal of residual nuclei following a cavitation event: a parametric study,” IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, vol. 62, no. 9, pp. 1605–1614, September 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Duryea AP, Cain CA, Roberts WW and Hall TL, “Removal of residual cavitation nuclei to enhance histotripsy fractionation of soft tissue,” IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, vol. 62, no. 12, pp. 2068–2078, December 2015. [PMC free article] [PubMed] [Google Scholar]
- [17].Duryea AP, Roberts WW, Cain CA, Tamaddoni HA and Hall TL, “Acoustic bubble removal to enhance SWL efficacy at high shock rate: an in vitro study,” Journal of Endourology, vol. 28, no. 1, pp. 90–95, January 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Alavi Tamaddoni H, Roberts WW, Duryea AP, Cain CA, and Hall TL, Journal of Endourology, vol. 30, no. 12, pp. 1321–1325, December 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pishchalnikov YA, McAteer JA, and Williams JC Jr., “Effect of firing rate on the performance of shock wave lithotriptors,” BJU International, vol. 102, no. 11, pp. 1681–6, December 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mettin R, Akhatov I, Parlitz U, Ohl CD, and Lauterborn W, “Bjerknes forces between small cavitation bubbles in a strong acoustic field,” Physical Review E, vol. 56, no. 3, pp. 2924–2931, September 1997. [Google Scholar]
- [21].Huber P, Jochle K, and Debus J, “Influence of shock wave pressure amplitude and pulse repetition frequency on the lifespan, size and number of transient cavities in the field of an electromagnetic lithotripter,” Physics in Medicine & Biology, vol. 43, no. 10, pp. 3113–28, October 1998. [DOI] [PubMed] [Google Scholar]
- [22].Pishchalnikov YA, McAteer JA, Pishchalnikova IV, Williams JC, Bailey MR, and Sapozhnikov OA, “Bubble proliferation in shock wave lithotripsy occurs during inertial collapse,” in 18th International Symposium on Nonlinear Acoustics, 2008, pp. 460–463. [Google Scholar]
- [23].Pishchalnikov YA, Williams JC, and McAteer JA, “Bubble proliferation in the cavitation field of a shock wave lithotripter,” Journal of the Acoustical Society of America, vol. 130, no. 2, pp. EL87–93, August 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Epstein PS and Plesset MS, “On the stability of gas bubbles in liquid-gas solutions,” The Journal of Chemical Physics, vol. 18, no. 11, pp. 1505–1509, 1950. [Google Scholar]
- [25].Pishchalnikov YA, McAteer JA, Williams JC Jr., Pishchalnikova IV and Vonderhaar RJ, “Why stones break better at slow shockwave rates than at fast rates: in vitro study with a research electrohydraulic lithotripter,” Journal of Endourology, vol. 20, no. 8, pp. 537–41, August 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Flynn HG, “Generation of transient cavities in liquids by microsecond pulses of ultrasound,” Journal of the Acoustical Society of America, vol. 72, no. 6, pp. 1926–32, December 1982. [Google Scholar]
- [27].Parsons JE, Cain CA, and Fowlkes JB, “Cost-effective assembly of a basic fiber-optic hydrophone for measurement of high amplitude therapeutic ultrasound fields,” Journal of the Acoustical Society of America, vol. 119, no. 3, pp. 1432–1440, March 2006. [DOI] [PubMed] [Google Scholar]
- [28].Bjerknes V, Fields of Force, 1906.
- [29].Bjerknes V, Die Kraftfelder, 1909.
- [30].Kornfeld M and Suvorov L, “On the destructive action of cavitation,” Journal of Applied Physics, vol. 15, no.6, pp. 495–506, 1944. [Google Scholar]
- [31].Blake FG, “Bjerknes Forces in Stationary Sound Fields,” The Journal of the Acoustical Society of America, vol. 21, no. 5, pp. 551–551, June 1949. [Google Scholar]
- [32].Neppiras EA, “Subharmonic and Other Low‐Frequency Emission from Bubbles in Sound‐Irradiated Liquids,” The Journal of the Acoustical Society of America, vol. 46, no. 3B, pp. 587–601, September 1969. [Google Scholar]
- [33].Crum LA and Eller AI, “Motion of Bubbles in a Stationary Sound Field,” The Journal of the Acoustical Society of America, vol. 48, no. 1B, pp. 181–189, July 1970. [Google Scholar]
- [34].Crum LA, “Bjerknes forces on bubbles in a stationary sound field,” The Journal of the Acoustical Society of America, vol. 57, no 6, pp. 1363–1370, June 1975. [Google Scholar]
- [35].Leighton TG, The Acoustic Bubble. San Diego, CA: Academic Press Inc, 1997. [Google Scholar]
- [36].Minnaert M, “XVI. On musical air-bubbles and the sounds of running water,” Philosophical Magazine Series 7, vol. 16, no. 104, pp. 235–248, August 1933. [Google Scholar]
- [37].Vlaisavljevich E, Lin K, Maxwell A, Warnez MT, Mancia L, Singh R, Putnam AJ, Fowlkes B, Johnsen E, Cain C, And Xu Z, “Effects of Ultrasound Frequency and Tissue Stiffness on the Histotripsy Intrinsic Threshold for Cavitation” Ultrasound in Medicine and Biology, vol. 41, no. 6, pp. 1651–1667, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Arvengas A, Herbert E, Cersoy S, Davitt K, and Caupin F, “Cavitation in Heavy Water and Other Liquids.” J Phys Chem B, vol. 115, pp. 14240–14245, October 2011. [DOI] [PubMed] [Google Scholar]
- [39].Herbert E, Balibar S, and Caupin F, “Cavitation Pressure in Water” Phys. Rev. E, vol. 74, pp. 041603, October 2006 [DOI] [PubMed] [Google Scholar]