Abstract
Firsthand and, to a lesser degree, secondhand tobacco smoking are considered the greatest causes of preventable illnesses and premature death worldwide. Firsthand and secondhand smoking have adverse consequences on the immune system, although these effects are not fully understood. A few serological studies have been done on firsthand and secondhand smokers in Saudi Arabia. The present study investigates the effects of firsthand and secondhand smoking on the immune system of randomly chosen male firsthand (50 subjects) and secondhand (50 subjects) cigarette smokers, residing in Jeddah, Saudi Arabia, with an age range of 20–40 years. Firsthand smokers were categorized according to the number of cigarettes smoked daily (frequency of smoking). Blood samples were collected and differential complete blood counts, cotinine concentrations, and antibodies (IgG, IgM, and IgA) concentrations were determined. Additionally, T, B, NK, CD4+ and CD8+ cells counts and percentages were determined. Compared to secondhand smokers, firsthand smokers showed a highly significantly higher mean cotinine concentration and a highly significantly lower mean IgA concentration. Additionally, Secondhand smokers had significantly higher mean lymphocyte count and CD4+/CD8+ ratio, and significantly lower mean basophil and NK cells counts. All other parameters showed no significant differences between firsthand and secondhand smokers and there were no differences between the frequency of smoking categories for the firsthand smokers. Therefore, The results show suggest that passive and active smoking have different immunological effects since IgA levels and some white blood cells counts were different in firsthand and secondhand smokers.
Keywords: Firsthand smokers, Secondhand smokers, Cigarettes, Cigarette smoking, Saudi Arabian males, Immune system, Cotinine, Antibodies, IgG, IgM, IgA, T cells, B cells, NK cells, CD4+ cells, CD8+ cells
Implications
Firsthand and secondhand tobacco smoking are widely prevalent in Saudi Arabia and rates of smoking continue to increase. For the last few decades, diseases and conditions associated with smoking have been increasing in Saudi Arabia. There is a lack of studies on the effects of smoking on the immune system in Saudi Arabia and thus this study fills a gap and provides much-needed results on these effects. In addition, this study draws attention to the need for strong recommendations and the implementation of educational and cessation programs to reduce the negative impact of smoking in Saudi Arabia.
Introduction
Tobacco use is a major cause of death worldwide, with 5 million firsthand (active) smokers and 600,000 secondhand (passive) smokers killed yearly [1], and a major contributor to health damage and preventable non-communicable illnesses. Addiction to nicotine, the major component of tobacco and cigarettes, is considered to be the most widespread addiction worldwide with cigarette smoking being the most common form of nicotine exposure. Cigarette smoking rates have been increasing in developing countries while at the same time decreasing in developed countries [2]. In addition, in the developed world increasingly stricter bans on smoking in public places are being enforced, leading to decreased secondhand smoke exposure for non-smokers. On the other hand, in developing countries such bans are non-existent or, if present, are usually not enforced nor adhered to. Additionally, many people in developing countries are not aware of the risks of firsthand smoking and, even more so, secondhand smoking.
Cigarette smoking is a major source of exposure to more than seven thousand carcinogens and toxic chemicals [3]. Cigarette smoking, including other forms of tobacco use, leads to a reduced life span; a reduced quality of life; an adversely affected immune system; and increased incidence of infections and diseases such as cancer, lung diseases, asthma, cardiovascular diseases, coronary heart disease, chronic obstructive pulmonary disease, and stroke [4–7]. Additionally, secondhand smokers are also adversely affected by exposure to smoke emitted by smokers at home and the workplace, thus, making them at an increased susceptibility, albeit to a lesser degree, to the same health ill-effects and diseases as smokers. Smoking not only affects health, but it also contributes to economic burdens to smokers and their countries alike through lost productivity of workers, increased sickness and hospitalizations, and wasteful spending of income.
Studies on the effects of firsthand and secondhand cigarette smoking and some specific components of cigarettes on humans and animals have shown different and sometimes contradictory effects [8, 9]. Firsthand and secondhand smoking were found to alter immunity causing inflammation, hypersensitivity, or/and a compromised immune response. Several components of cigarette smoke have been shown to affect immunity and its mediators in in vitro and in vivo studies, although there is no general agreement on these effects. Mucosal surfaces are dramatically compromised by cigarette smoke leading to increased incidence rates of respiratory infections and diseases in smokers [10, 11]. Smoking has been shown to modulate both the innate and acquired immunities through effects on different types of immune cells and molecules [12, 13] although there is no general consensus on the type of these effects.
Saudi Arabia is ranked fourth in tobacco imports worldwide [14]. Epidemiological studies have shown that smoking rates are increasing in Saudi Arabia among both females and males. Therefore, the aim of this study was to determine the effects of firsthand and secondhand cigarette smoking on some biochemical and immunological parameters in healthy males in the Saudi Arabian general population. This would help in determining the effects of firsthand and secondhand smoking on general health and the immune system of the local population, thus aiding in providing smokers with a better picture of the health disadvantages they are exposing themselves to and giving them more incentive to stop smoking.
Materials and Methods
Subjects and Frequency of Smoking Categorization
For this study, 50 male firsthand cigarette smokers and 50 male secondhand cigarette smokers with an age range of 20–40 years were used. All subjects were randomly chosen from blood donors to the blood bank at King Abdulaziz Hospital, Makkah, Saudi Arabia. To be included in the study, a subject must be self-proclaimed to be a firsthand or secondhand smoker. In addition, all subjects were not taking any medications, nor suffering from any chronic diseases, diabetes, immune diseases, blood pressure, hereditary diseases, anemia, heart disease, or allergic diseases. Each subject signed a consent form and filled a questionnaire to assess his health state and any lifestyle factors that may influence the parameters. Ethical approval for the study was obtained from King Abdulaziz University.
Firsthand smokers were divided into four groups based on the number of cigarettes smoked per day as follows: light smokers (10 cigarettes or less), moderate smokers (between 11 and 20 cigarettes), heavy smokers (between 21 and 30 cigarettes), and finally very heavy smokers (31 or more cigarettes).
Blood Collection
Blood samples were collected from the subjects in plain vacutainer tubes, after which serum was separated and stored at − 80 °C for later determination of antibodies and cotinine concentrations. Whole blood samples were collected in EDTA (ethylene diamine tetra-acetic acid) vacutainer tubes and stored in a cooler for about 4–5 h before the differential complete blood count and flow cytometric analyses.
Determination of Differential Complete Blood Counts, and Concentrations of Serum Cotinine, IgG, IgM, and IgA
Differential complete blood counts were done at King Abdulaziz Hospital, Makkah, Saudi Arabia on an automated XT-2000ί Hematology Analyzer (Sysmex Corporation, Kobe, Japan). The chemicals used were the EPK Cellpack (Sysmex Corporation, Kobe, Japan). A Calbiotech Cotinine ELISA Kit (Calbiotech, Austin, Texas, USA) was used, according to the manufacturer’s instructions, to measure the serum concentrations of cotinine. The absorbances were read using a Stat Fax 2100 Microplate Reader (Awareness Technology, Ramsey, Minnesota, USA), at King Abdulaziz University Hospital, Jeddah, Saudi Arabia. Serum concentrations of the IgG, IgM, and IgA immunoglobulins were determined using the ARCHITECT c8000 System (Abbott Diagnostics, Japan), at King Abdulaziz Medical City, Jeddah, Saudi Arabia, using the manufacturer’s recommended reagents (Abbott, Green Oaks, Illinois, USA).
Determination of Lymphocyte Subsets Counts
The determination of whole blood lymphocyte subsets was done at King Abdulaziz Medical City, Jeddah, Saudi Arabia, using a BD FACSCanto II flow cytometer (Becton–Dickinson Company, San Jose, CA, USA) and the BD Multitest 6-color TBNK reagent (BD Biosciences, San Jose, CA, USA). The concentrations of B, T and NK lymphocytes were detected by determining the accessory molecules (CD markers) on their surfaces.
Statistical Methods
The mean (μ), standard deviation (± SD), standard error of the mean (± SE), and the P value for the differences between the groups were determined for all parameters and the data were graphically represented using the SPSS v16 statistical program.
For the normally distributed and homogeneous parameters, the t test was used to test for the significance of the differences between the firsthand and secondhand smokers for the parameters, while the ANOVA one-way test was used to test for the significance of the differences between the frequency of firsthand smoking groups for each parameter measured. For the parameters that showed a non-normal distribution, the Mann–Whitney U test was used for the comparison between the firsthand and secondhand smokers, while the Kruskal–Wallis H test was used to test for the significance of the differences between the frequency of smoking groups. The resulting P values demonstrate significance or non-significance between groups as follows: P > 0.05 is a non-significant (NS) difference, 0.01 ≤ P ≤ 0.05 is a significant (S) difference, and P < 0.01 is a highly significant (HS) difference.
Results
Subjects and Frequency of Smoking Categorization
A total of 100 male subjects were randomly collected for this research, with 50 being firsthand smokers (46% Saudi, 54% non-Saudi) and 50 being secondhand smokers (48% Saudi, 52% non-Saudi). The mean age of the firsthand smokers (mean ± SE = 29.20 ± 0.72 years) was higher than that for the secondhand smokers (mean ± SE = 27.06 ± 0.77). The box-plot comparison of ages of the firsthand and secondhand smokers is shown in Fig. 1.
Fig. 1.
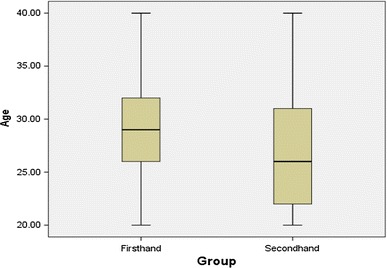
Box-plot comparison of ages for the firsthand (n = 50) and secondhand (n = 50) smokers
Firsthand smokers were divided into four groups based on the number of cigarettes smoked per day by each subject, as shown in Fig. 2. The four groups were: light smokers (control) (frequency, percent: 22, 44%), moderate smokers (16, 32%), heavy smokers (6, 12%), and very heavy smokers (6, 12%).
Fig. 2.
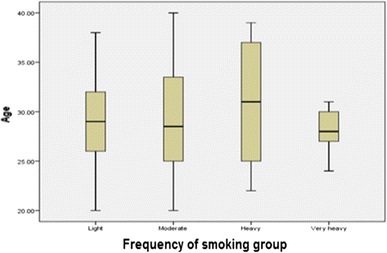
Box-plot comparison of the frequency of firsthand smoking categories (number of subjects: light = 22, moderate = 16, heavy = 6, and very heavy = 6)
Cotinine concentrations
The mean serum cotinine concentration (Table 1) for the firsthand smokers, using the Mann–Whitney U test, was highly significantly higher than the mean concentration for the secondhand smokers. As for the relationship between the frequency of smoking categories and mean cotinine concentrations, the Kruskal–Wallis test (Table 2) showed no significant relationship.
Table 1.
Descriptive statistics and test of significance for the differences between firsthand and secondhand smokers for cotinine and immunoglobulins concentrations, and differential white blood cells counts
| Cell counts | Group | Minimum | Maximum | Mean | ± SE | P value |
|---|---|---|---|---|---|---|
| WBC* (103/µL) | Firsthand | 3.11 | 10.32 | 6.61 | 0.24 | 0.744NS |
| Secondhand | 3.18 | 10.90 | 6.49 | 0.27 | ||
| Neutrophils* (103/µL) | Firsthand | 0.99 | 5.37 | 3.13 | 0.17 | 0.381NS |
| Secondhand | 1.02 | 6.31 | 3.36 | 0.20 | ||
| Lymphocytes* (103/µL) | Firsthand | 1.49 | 4.41 | 2.64 | 0.09 | 0.020S |
| Secondhand | 1.36 | 4.41 | 2.33 | 0.09 | ||
| Monocytes* (103/µL) | Firsthand | 0.24 | 0.86 | 0.56 | 0.02 | 0.307NS |
| Secondhand | 0.23 | 1.09 | 0.52 | 0.03 | ||
| Eosinophils** (103/µL) | Firsthand | 0.04 | 1.30 | 0.26 | 0.03 | 0.674NS |
| Secondhand | 0.04 | 0.86 | 0.25 | 0.03 | ||
| Basophils** (103/µL) | Firsthand | 0.01 | 0.09 | 0.03 | 0.00 | 0.016S |
| Secondhand | 0.01 | 0.13 | 0.04 | 0.00 | ||
| Cotinine** (ng/mL) | Firsthand | 2.00 | 145.00 | 107.36 | 6.59 | 0.000HS |
| Secondhand | 0.00 | 145.00 | 53.71 | 9.47 | ||
| IgG* (g/L) | Firsthand | 5.16 | 16.89 | 11.92 | 0.34 | 0.513NS |
| Secondhand | 6.43 | 17.97 | 12.24 | 0.36 | ||
| IgA* (g/L) | Firsthand | 0.74 | 3.37 | 1.86 | 0.09 | 0.005HS |
| Secondhand | 1.14 | 5.10 | 2.37 | 0.15 | ||
| IgM** (g/L) | Firsthand | 0.22 | 1.85 | 0.82 | 0.06 | 0.537NS |
| Secondhand | 0.41 | 2.07 | 0.88 | 0.06 |
*T test was used for the significance test
**Mann–Whitney U test was used for the significance test
SE standard error, HS highly significant (P < 0.01), S significant (P ≤ 0.05), NS not significant (P > 0.05)
Firsthand smokers n = 50 and secondhand smokers n = 50
Table 2.
Descriptive statistics and test of significance for the relationships between the frequency of smoking and cotinine and immunoglobulins concentrations
| Parameter | Group | N | Minimum | Maximum | Mean | ± SE | P value |
|---|---|---|---|---|---|---|---|
| Cotinine** (ng/mL) | Light | 17 | 2.00 | 140.00 | 112.97 | 8.48 | 0.986NS |
| Moderate | 12 | 26.50 | 139.00 | 102.46 | 12.80 | ||
| Heavy | 4 | 3.50 | 140.00 | 99.25 | 32.25 | ||
| Very heavy | 6 | 22.00 | 145.00 | 106.67 | 17.92 | ||
| IgG* (g/L) | Light | 22 | 5.16 | 14.66 | 12.01 | 0.46 | 0.976NS |
| Moderate | 16 | 6.78 | 16.89 | 11.69 | 0.68 | ||
| Heavy | 6 | 8.84 | 16.14 | 12.02 | 1.13 | ||
| Very heavy | 6 | 9.39 | 16.63 | 12.09 | 1.09 | ||
| IgA* (g/L) | Light | 22 | 0.82 | 3.27 | 1.94 | 0.13 | 0.789NS |
| Moderate | 16 | 0.74 | 3.37 | 1.77 | 0.17 | ||
| Heavy | 6 | 0.78 | 2.44 | 1.71 | 0.29 | ||
| Very heavy | 6 | 1.28 | 3.13 | 1.96 | 0.26 | ||
| IgM** (g/L) | Light | 22 | 0.32 | 1.77 | 0.83 | 0.10 | 0.919NS |
| Moderate | 16 | 0.22 | 1.85 | 0.86 | 0.10 | ||
| Heavy | 6 | 0.49 | 1.08 | 0.72 | 0.09 | ||
| Very heavy | 6 | 0.41 | 1.01 | 0.79 | 0.09 |
*ANOVA one-way test was used for the significance test
**Kruskal–Wallis test was used for the significance test
SE standard error, NS not significant (P > 0.05)
Immunoglobulin concentrations
The mean concentrations of IgG and IgM antibodies (Table 1) for the firsthand and secondhand smokers showed no significant differences using the t-test for IgG and the Mann–Whitney U test for IgM. Using the t-test, the mean IgA concentration for the firsthand smokers was highly significantly lower than the mean concentration for the secondhand smokers. On the other hand, the mean IgG and IgA concentrations, using the ANOVA one-way test (Table 2), and the mean IgM concentrations, using the Kruskal–Wallis test, did not show any significant differences between the frequency of smoking categories.
Differential White Blood Cell Counts
The mean counts of the total white blood cells (WBC), neutrophils, monocytes (using the t-test), and eosinophils (using the Mann–Whitney U test) for the firsthand and secondhand smokers showed no significant differences (Table 1). Using the t-test, the mean lymphocyte cell counts for the firsthand smokers was significantly higher than the mean count for the secondhand smokers. The mean basophil cell count for the firsthand smokers, using the Mann–Whitney U test, was significantly lower than that for the secondhand smokers. As for the frequency of smoking categories (Table 3), there were no significant differences between the categories for the mean WBC, neutrophil, lymphocyte, and monocyte cells counts, using the ANOVA one-way test, nor for the mean eosinophil and basophil counts, using the Kruskal–Wallis test.
Table 3.
Descriptive statistics and test of significance for the relationships between the frequency of smoking and total and differential WBC counts
| Cell counts (103/µL) | Group | Minimum | Maximum | Mean | ± SE | P value |
|---|---|---|---|---|---|---|
| WBC* | Light | 4.54 | 10.32 | 6.22 | 0.33 | 0.368NS |
| Moderate | 3.11 | 9.39 | 7.10 | 0.47 | ||
| Heavy | 4.31 | 9.44 | 7.09 | 0.83 | ||
| Very heavy | 4.64 | 8.32 | 6.25 | 0.55 | ||
| Neutrophils* | Light | 1.36 | 4.84 | 2.83 | 0.21 | 0.187NS |
| Moderate | 0.99 | 5.37 | 3.62 | 0.31 | ||
| Heavy | 1.73 | 5.21 | 3.25 | 0.63 | ||
| Very heavy | 1.94 | 4.43 | 2.80 | 0.42 | ||
| Lymphocytes* | Light | 1.71 | 4.41 | 2.64 | 0.15 | 0.669NS |
| Moderate | 1.49 | 3.80 | 2.58 | 0.17 | ||
| Heavy | 2.01 | 3.65 | 2.93 | 0.24 | ||
| Very heavy | 2.04 | 2.83 | 2.49 | 0.13 | ||
| Monocytes* | Light | 0.24 | 0.83 | 0.51 | 0.03 | 0.385NS |
| Moderate | 0.25 | 0.85 | 0.60 | 0.05 | ||
| Heavy | 0.36 | 0.86 | 0.62 | 0.09 | ||
| Very heavy | 0.36 | 0.70 | 0.54 | 0.05 | ||
| Eosinophils** | Light | 0.04 | 0.78 | 0.20 | 0.03 | 0.364NS |
| Moderate | 0.04 | 1.08 | 0.28 | 0.07 | ||
| Heavy | 0.12 | 0.47 | 0.27 | 0.05 | ||
| Very heavy | 0.16 | 1.30 | 0.39 | 0.18 | ||
| Basophils** | Light | 0.01 | 0.07 | 0.03 | 0.00 | 0.578NS |
| Moderate | 0.01 | 0.09 | 0.03 | 0.01 | ||
| Heavy | 0.02 | 0.06 | 0.03 | 0.00 | ||
| Very heavy | 0.01 | 0.03 | 0.02 | 0.00 |
*ANOVA one-way test was used for the significance test
**Kruskal–Wallis test was used for the significance test
SE standard error, NS not significant (P > 0.05)
Number of subjects: light = 22, moderate = 16, heavy = 6, and very heavy = 6
Percentages of Lymphocyte Subtypes
Using the t-test (Table 4), the mean total T, CD4+, CD8+, and total B cells percentages for the firsthand and secondhand smokers showed no significant differences. On the other hand, the mean NK cell percentages, using the t-test, were significantly lower for the firsthand smokers compared to the secondhand smokers. For the relationships between the frequency of smoking categories and the mean total T, CD4+, CD8+, total B, and NK cells percentages, the ANOVA one-way test was used (Table 5) and it showed no significant differences.
Table 4.
Descriptive statistics and test of significance for the differences between firsthand and secondhand smokers for the percentages and counts of sub-lymphocytes using the t-test
| Cell | Group | Minimum | Maximum | Mean | ± SE | P value |
|---|---|---|---|---|---|---|
| T % | Firsthand | 57.20 | 82.22 | 73.16 | 0.79 | 0.057NS |
| Secondhand | 56.21 | 85.53 | 70.84 | 0.91 | ||
| T counts | Firsthand | 604.49 | 2972.13 | 1824.20 | 83.53 | 0.093NS |
| Secondhand | 991.53 | 3177.07 | 1649.80 | 59.91 | ||
| CD4+ % | Firsthand | 28.81 | 53.23 | 40.52 | 0.91 | 0.271NS |
| Secondhand | 27.09 | 52.18 | 39.16 | 0.83 | ||
| CD4+ counts | Firsthand | 397.48 | 2104.38 | 1011.30 | 53.09 | 0.113NS |
| Secondhand | 477.80 | 1427.34 | 910.34 | 34.00 | ||
| CD8+ % | Firsthand | 14.52 | 42.01 | 28.71 | 0.89 | 0.054NS |
| Secondhand | 18.98 | 43.06 | 31.12 | 0.86 | ||
| CD8+ counts | Firsthand | 142.76 | 1289.92 | 716.98 | 37.61 | 0.872NS |
| Secondhand | 431.10 | 1779.68 | 725.20 | 34.01 | ||
| B % | Firsthand | 5.64 | 24.38 | 15.23 | 0.65 | 0.647NS |
| Secondhand | 5.78 | 23.60 | 14.83 | 0.59 | ||
| B counts | Firsthand | 129.02 | 1010.62 | 381.64 | 26.62 | 0.417NS |
| Secondhand | 127.25 | 995.14 | 353.30 | 22.37 | ||
| NK % | Firsthand | 2.49 | 26.74 | 10.87 | 0.73 | 0.010S |
| Secondhand | 3.02 | 28.35 | 13.66 | 0.78 | ||
| NK counts | Firsthand | 28.14 | 1005.59 | 266.35 | 22.43 | 0.061NS |
| Secondhand | 77.34 | 858.51 | 331.02 | 25.74 | ||
| CD4+/CD8+ ratio | Firsthand | 0.82 | 3.23 | 1.52 | 0.08 | 0.038S |
| Secondhand | 0.77 | 2.36 | 1.32 | 0.05 |
SE standard error, S significant (P ≤ 0.05), NS not significant (P > 0.05)
Firsthand smokers n = 50 and secondhand smokers n = 50
Table 5.
Descriptive statistics and test of significance for the relationships between the frequency of smoking and the percentages of the types of lymphocytes using the ANOVA one-way test
| Cell % | Group | Minimum | Maximum | Mean | ± SE | P value |
|---|---|---|---|---|---|---|
| T cells | Light | 57.20 | 81.63 | 72.29 | 1.44 | 0.752NS |
| Moderate | 65.23 | 82.22 | 73.38 | 1.17 | ||
| Heavy | 69.67 | 82.09 | 74.78 | 1.90 | ||
| Very heavy | 66.22 | 78.82 | 74.15 | 1.82 | ||
| CD4+ cells | Light | 28.81 | 50.76 | 39.82 | 1.27 | 0.779NS |
| Moderate | 30.41 | 53.23 | 41.68 | 1.83 | ||
| Heavy | 35.32 | 51.80 | 41.32 | 2.46 | ||
| Very heavy | 31.35 | 51.17 | 39.18 | 2.87 | ||
| CD8+ cells | Light | 14.52 | 42.01 | 28.43 | 1.50 | 0.816NS |
| Moderate | 18.32 | 34.81 | 27.96 | 1.19 | ||
| Heavy | 21.41 | 41.22 | 30.25 | 2.75 | ||
| Very heavy | 17.88 | 37.52 | 30.21 | 3.08 | ||
| B cells | Light | 5.64 | 24.38 | 15.42 | 1.17 | 0.977NS |
| Moderate | 9.02 | 19.28 | 15.12 | 0.86 | ||
| Heavy | 7.64 | 20.65 | 15.55 | 2.16 | ||
| Very heavy | 8.98 | 21.51 | 14.53 | 1.75 | ||
| NK cells | Light | 3.22 | 26.74 | 11.55 | 1.33 | 0.796NS |
| Moderate | 2.49 | 19.82 | 10.67 | 1.20 | ||
| Heavy | 4.66 | 14.84 | 9.21 | 1.40 | ||
| Very heavy | 6.56 | 13.77 | 10.52 | 1.30 |
SE standard error, NS not significant (P > 0.05)
Number of subjects: light = 22, moderate = 16, heavy = 6, and very heavy = 6
Counts of Lymphocyte Subtypes
Using the t-test (Table 4), the mean total T, CD4+, CD8+, total B, and NK cells counts for the firsthand and secondhand smokers showed no significant differences, while the mean CD4+/CD8+ counts ratio for the firsthand smokers was significantly higher than the mean ratio for the secondhand smokers. The mean counts for the total T, CD4+, CD8+, total B, NK cells and CD4+/CD8+ cells counts, using the ANOVA one-way test (Table 6), showed no significant differences between the frequency of smoking groups.
Table 6.
Descriptive statistics and test of significance for the relationships between the frequency of smoking and the counts of the types of lymphocytes using the ANOVA one-way test
| Cell counts | Group | Minimum | Maximum | Mean | ± SE | P value |
|---|---|---|---|---|---|---|
| T cells | Light | 627.95 | 2972.13 | 1840.10 | 132.08 | 0.915NS |
| Moderate | 604.49 | 2935.05 | 1763.80 | 165.67 | ||
| Heavy | 872.05 | 2578.10 | 1936.00 | 253.55 | ||
| Very heavy | 1440.94 | 2059.69 | 1787.10 | 104.69 | ||
| CD4+ cells | Light | 461.50 | 2104.38 | 1016.90 | 86.47 | 0.971NS |
| Moderate | 397.48 | 1629.21 | 1004.20 | 102.24 | ||
| Heavy | 592.09 | 1373.55 | 1063.70 | 128.08 | ||
| Very heavy | 693.81 | 1464.24 | 957.68 | 117.69 | ||
| CD8+ cells | Light | 142.76 | 1271.87 | 720.55 | 56.58 | 0.756NS |
| Moderate | 191.91 | 1289.92 | 674.83 | 71.94 | ||
| Heavy | 244.77 | 1127.73 | 815.78 | 129.37 | ||
| Very heavy | 511.56 | 1009.97 | 717.52 | 71.77 | ||
| B cells | Light | 160.42 | 1010.62 | 390.40 | 45.22 | 0.853NS |
| Moderate | 129.02 | 631.59 | 358.09 | 36.97 | ||
| Heavy | 144.84 | 742.09 | 433.19 | 103.36 | ||
| Very heavy | 168.85 | 615.45 | 360.77 | 61.45 | ||
| NK cells | Light | 79.09 | 1005.59 | 288.34 | 44.01 | 0.867NS |
| Moderate | 28.14 | 416.29 | 250.39 | 29.91 | ||
| Heavy | 95.26 | 436.10 | 244.91 | 51.02 | ||
| Very heavy | 162.07 | 344.69 | 249.76 | 29.52 | ||
| CD4+/CD8+ ratio | Light | 0.82 | 3.23 | 1.53 | 0.13 | 0.968NS |
| Moderate | 0.87 | 2.90 | 1.57 | 0.14 | ||
| Heavy | 0.91 | 2.42 | 1.46 | 0.22 | ||
| Very heavy | 0.84 | 2.86 | 1.45 | 0.31 |
SE standard error, NS not significant (P > 0.05)
Number of subjects: light = 22, moderate = 16, heavy = 6, and very heavy = 6
Discussion
To determine how cigarette smoking affects immunity differently in firsthand and secondhand smokers and whether the intensity of smoking leads to different effects, counts of white blood cells important in innate immunity (neutrophils, monocytes, eosinophils, basophils and NK cells) and acquired immunity (T and B lymphocytes) were determined. Additionally, the effects of firsthand and secondhand smoking on humoral acquired immunity were measured by determining the concentrations of IgG, IgM, and IgA antibodies; and the counts and percentages of B cells. The effects on cellular immunity were assessed by determining the counts and percentages of total T, CD4+, and CD8+ cells.
In comparing the ages of firsthand and secondhand smokers, firsthand smokers had a higher median while secondhand smokers had a lower first quartile. Thus, more secondhand smokers are younger in age. As for the frequency of smoking for the firsthand smokers group, heavy smokers had the highest median and very heavy smokers had the lowest median. Therefore, on average, heavy smokers were the oldest of the subjects while very heavy smokers were the youngest. Moderate smokers had the widest range while very heavy smokers had the narrowest range. Thus, moderate smokers were widespread among all ages unlike the very heavy smokers who were mainly younger smokers.
The intensity of smoking and the extent of exposure to tobacco smoke is most commonly estimated by measuring the concentration of cotinine, a metabolite of nicotine found in body fluids with a long half-life, rather than measuring nicotine concentration since it has a shorter half-life [15, 16]. The results showed that the mean cotinine concentrations were highly significantly higher (P = 0.000) for firsthand smokers (mean ± SE: 107.36 ± 6.59) compared to the secondhand smokers group (53.71 ± 9.47). The findings of the current study are consistent with those of other studies [15, 16] that found higher values of cotinine in smokers compared to secondhand smokers or non-smokers. On the other hand, the mean cotinine concentrations for the frequency of smoking groups were very close to each other and not significantly different (P = 0.986), which is unexpected. Contrary to these findings, Benowitz [16] found increased cotinine levels with increased intensity of smoking. Our results showed that most firsthand smokers were light (44%) and moderate smokers (32%), which is a total of 76%, leaving a total of 24% for heavy and very heavy smokers. Thus, there may have been some false self-reporting with probably heavy or very heavy smokers underestimating or underreporting the number of cigarettes they smoked daily since it would be expected that there would be some significant differences between the cotinine concentrations for the frequency of smoking categories and that the subjects would be more evenly distributed among the groups.
Most studies used below for comparisons with our results compared firsthand smokers with non-smokers. We were not able to find many studies that compared firsthand and secondhand smokers for the parameters investigated here. Non-smokers groups probably contain many secondhand smokers, unless specifically stated to the contrary, since most people are exposed to secondhand smoke. Therefore, it is justifiable to use such studies for comparisons with our findings.
There were no significant differences (P = 0.513, and P = 0.537, respectively) between firsthand and secondhand smokers for mean serum IgG and IgM concentrations. The mean serum IgA concentration for firsthand smokers (1.86 ± 0.09) was highly significantly lower (P = 0.005) compared with secondhand smokers (2.37 ± 0.15). These findings of unaffected IgG and IgM concentrations agree with some other findings [17] but are contradictory to many other studies that found lower IgG [8, 18–20] and IgM levels [8, 20], or higher levels of the antibodies [21] in smokers compared to non-smokers. The lower IgA level in firsthand smokers agrees with some research studies [20, 22, 23] but contradicts the findings of other research studies that found higher levels [8, 22] or no change in the levels [17] in smokers compared to non-smokers. Our finding of lower mean serum IgA level for firsthand smokers may explain the increased susceptibility of smokers to upper respiratory tract infections. The absence of differences in mean IgG and IgM levels between firsthand and secondhand smokers may be due to both firsthand and secondhand smokers being similarly affected by cigarettes. That is, IgG and IgM levels are compromised to the same extent or, probably unlikely, not affected by smoking. To determine the effects of firsthand and secondhand smoking on IgG and IgM, a comparison must be made between the firsthand and secondhand groups with a group of matched non-smokers that are not exposed to secondhand smoke.
Comparing firsthand and secondhand smokers for the differential white blood cells counts, there were no significant differences (P = 0.744, P = 0.381, P = 0.307, and P = 0.674, respectively) for the mean cell counts for total white blood cells, neutrophils, monocytes, and eosinophils. Findings of other researchers on the levels of total serum white blood cells and their types in smokers are contradictory with each other and not all are in agreement with the findings of the current study. Researchers [8, 12, 24–34] have shown higher counts of total white blood cells in smokers compared to non-smokers. Additionally, these results contradict findings by other researchers of higher counts of neutrophils [24, 29–36], monocytes and eosinophils [24, 31–34] in smokers compared to non-smokers. The result of no change in monocyte levels agrees with the findings of the study by Tollerud et al. [34].
The mean lymphocyte count for firsthand smokers (2.64 ± 0.09) was significantly higher (P = 0.020) than for secondhand smokers (2.33 ± 0.09), while the mean basophil count for firsthand smokers (0.03 ± 0.00) was significantly lower (P = 0.016) compared to secondhand smokers (0.04 ± 0.00). This is in agreement with the results of several researchers [24, 25, 29–34] who found higher lymphocyte counts in smokers compared to non-smokers, but is in disagreement with other research studies [25, 33, 36] that showed lower lymphocyte counts in smokers. As for the resultant lower mean basophil count for firsthand smokers, the results are not consistent with other research studies [24, 31, 33] that found significantly higher levels in smokers compared to non-smokers.
White blood cells increase in numbers in the case of an infection or invasion of a pathogen. In addition, these cells increase in some unhealthy states or lifestyles, such as smoking, obesity, diabetes, and other inflammatory states. Studies have shown that smoking leads to a non-specific inflammatory state which may be due to increased counts of cells involved in inflammation or in mediating it, such as lymphocytes, basophils and monocytes. Therefore, the current results of higher lymphocyte counts in firsthand smokers and higher basophil counts in secondhand smokers is in line with these findings.
There were no significant differences (P = 0.057, P = 0.271, P = 0.054, and P = 0.647, respectively) between firsthand and secondhand smokers for mean percentages for total T (CD3+), CD4+, CD8+, and B cells (CD19+). On the other hand, the mean NK cell (CD56 + CD16+) percentages were significantly lower (P = 0.010) for firsthand smokers (10.87 ± 0.73) compared to secondhand smokers (13.66 ± 0.78). The mean cell counts for total T, CD4+, CD8+, B, and NK cells were not significantly different (P = 0.093, P = 0.113, P = 0.872, P = 0.417, and P = 0.061, respectively) between firsthand and secondhand smokers. As for the mean CD4+/CD8+ ratio, it was significantly higher (P = 0.038) for firsthand smokers (1.52 ± 0.08) compared to secondhand smokers (1.32 ± 0.05).
Contrary to the above results, numerous in vitro and in vivo studies in both humans and mice have shown tobacco smoke to lower counts and percentages of T and B lymphocytes [34, 37–42] or increase T cell percentages [41, 43, 44] compared to non-smokers. In addition, contradictory to the findings here, other studies observed lower percentages of CD4+ cells [8, 37, 38, 41, 43, 44], higher CD4+ cell counts and percentages [34, 41–44], and higher percentages of CD8+ cells [34, 41–46] in smokers compared to non-smokers. In agreement with the current results, some studies found higher CD4+/CD8+ ratios [34, 42] and lower NK cell counts [34] in smokers compared with non-smokers, and lower NK cell percentages in secondhand smokers compared to non-smokers [47]. A study [34] found unchanged T, B, and CD8 + cell counts for smokers compared to non-smokers.
For the categorization of firsthand smokers into frequencies of smoking, none of the measured parameters showed any differences between the groups. Thus, the number of cigarettes smoked by firsthand smokers did not correlate with any parameter. Not many studies were found on the effects of the intensity of smoking on the parameters studied here. Some research studies [28, 48] found that WBC counts increased significantly with increasing intensity of smoking. Studies [18, 49] also showed a decrease in IgG levels with increased levels of firsthand smoking. A study observed [42] that light to moderate smokers had a significant increase in T, CD4+ and CD8+ cells counts and light smokers showed increased CD4+/CD8+ ratio due to increased CD4+ cell counts. Some studies [41, 43, 44] found lower CD4+ cell counts in heavy smokers.
Conclusions and Recommendations
In conclusion, firsthand smokers had a significantly higher mean cotinine level, lymphocyte count, and CD4+/CD8+ ratio compared to secondhand smokers. In addition, firsthand smokers had a significantly lower mean IgA concentration, basophil count, and NK cell count compared to secondhand smokers. The higher cotinine concentration in firsthand smokers is expected. Cotinine is used to distinguish smokers from non-smokers since it is usually higher in smokers and heavy secondhand smokers but not light to moderate secondhand smokers or non-smokers.
The higher mean lymphocyte count in firsthand smokers could be an indication that the immune system of the subjects is sensitized by the effects of cigarettes or there is an inflammatory reaction since some types of lymphocytes are associated with and mediators of inflammation and their numbers increase with increasing inflammation. Thus, this increase may be due to the immune system’s response to the toxic effects and components of cigarettes and its attempt to overcome the resulting damage.
For secondhand smokers, mean IgA concentration, basophil count, and NK cell percentage were higher than those in firsthand smokers. The higher levels of these parameters may be an indication that the immune system is affected due to the exposure to secondhand smoke. The higher counts of NK cells, which are involved in innate immunity, may be due to the general alteration of the immune system or a specific reaction to cells adversely affected by secondhand smoke. Both IgA and basophils are important in allergic response and respiratory infections and diseases. Therefore, it seems that secondhand smokers are more susceptible to hypersensitivity reactions due to their exposure to the smoke of firsthand smokers. The major protective antibody in mucosal surfaces and the respiratory system is IgA, although not all studies agree on it being affected in smokers. In addition to IgA, basophils are also involved in the protection of mucosal surfaces, and against respiratory and mucosal antigens. Therefore, their higher levels may be due to inflammatory effects of secondhand smoke on mucosal surfaces or an attempt of these surfaces to counteract the effects of secondhand smoke, or, in other words, to mount an immune response against it. Basophils, in particular, are involved in allergies and asthma, which appear at higher rates with increased inhalation of cigarette smoke. When basophils are activated, they also degranulate and release histamine and other inflammatory mediators therefore leading to increased inflammation in smokers, as proven by many research studies.
It is not surprising that IgA was the only antibody affected in the subjects since, as mentioned above, it is the antibody that is responsible for the protection of mucosal surfaces against pathogens and it is the major antibody in mucosal surfaces. Thus, the lower IgA concentration in firsthand smokers may be due to the compromise of immunity in the respiratory system due to smoking. This is further corroborated by the observed lower basophil counts. On the other hand, the higher counts of basophils in secondhand smokers may mean that they are experiencing a reaction to the cigarette smoke that is allergic or asthmatic in nature since basophils are mainly involved in mediating respiratory allergies and asthma and cause inflammation. In addition, the higher mean IgA concentration in secondhand smokers relative to firsthand smokers may be an indication that there is an active response against the inhaled smoke in secondhand smokers. Therefore, firsthand smokers may be responding to cigarette smoke differently than secondhand smokers, and an important factor is that each type of exposure (active or passive) to cigarette smoke may lead to varying effects on the immune system.
On the other hand, the mean CD4+/CD8+ ratio for firsthand smokers was higher than for secondhand smokers. A higher CD4+/CD8+ ratio is an indication of a more active immune system in firsthand smokers compared to secondhand smokers. Therefore, it may be that in firsthand smokers the immune system is activated and is fighting the deleterious effects of smoking on the body and/or the immune system. This is especially problematic since an enhanced immune response is a natural response to the presence of an antigen, but in this case there is no antigen and thus this may lead to damaging effects on the body.
The frequency of smoking groups must not have been accurate since none of the parameters gave any differences between the groups, including the mean cotinine concentrations which are expected to be different for the different groups. This is probably due to false self-reporting which may be due to the social stigma of smoking perceived by some people in Saudi Arabia.
In conclusion, the data suggest that both firsthand and secondhand smoking lead to some different immunological effects, although these effects are not completely understood. It is recommended that more studies be carried out on both firsthand and secondhand smokers at different ages using other parameters along with comparative studies on females.
Funding
Partial funding for this study was provided through a grant from King Abdulaziz City of Science and Technology.
Conflict of interest
Both authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.World Health Organization (WHO), Tobacco Fact Sheet. 2016 (http://www.who.int/mediacentre/factsheets/fs339/en/).
- 2.Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res. 2015;4(4):327–338. doi: 10.3978/j.issn.2218-6751.2015.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Cancer Institute, Annual report to the nation finds cancer death rates still on the decline: progress in cancer treatment varies by disease. 2005 NIH publication. Department of Health and Human Services.
- 4.Mahassni SH, Alajlany KA. Levels of some electrolytes and glucose in Saudi water pipe smokers. J Health Res Rev. 2017;4(1):30–34. doi: 10.4103/2394-2010.199330. [DOI] [Google Scholar]
- 5.Mahassni SH, Bukhari AA, Bukhari MA, Al-khathami AS. Dyslipidemia and hypertension in Saudi male cigarette smokers. J Basic Appl Res Int. 2016;19(1):30–37. [Google Scholar]
- 6.Sumanasekera W, Nethery W, Nguyen S. Nicotine in cigarette smoke: addiction, health effects, detection methods, and smoking cessation. J Addict Behav Ther Rehabil. 2016;5:2. doi: 10.4172/2324-9005.1000154. [DOI] [Google Scholar]
- 7.Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9(5):377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JD, Houchens DP, Kluwe WM, Craig DK, Fisher GL. Effects of mainstream and environmental tobacco smoke on the immune system in animals and humans: a review. CRC Crit. Rev. Toxicol. 1990;20(5):369–395. doi: 10.3109/10408449009089870. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez JW, Kirlin WG, Wirsiy YG, Matheravidathu S, Hodge TW. Maternal exposure to benzo [a] pyrene alters development of T lymphocytes in offspring. Immunopharm. Immunot. 1999;21(2), 379–396. [DOI] [PubMed]
- 10.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2(5), 372–377. [DOI] [PubMed]
- 11.Li L, Holian A. Acrolein: a respiratory toxin that suppresses pulmonary host defense. Rev Environ Health. 1997;13(1–2):99–108. [PubMed] [Google Scholar]
- 12.Asif M, Karim S, Umar Z, Malik A, Ismail T, Chaudhary A, et al. Effect of cigarette smoking based on hematological parameters: comparison between male smokers and non-smokers. Türk Biyokimya Dergisi [Turk J Biochem]. 2013;38(1):75–80. [Google Scholar]
- 13.Finkelstein EI, Nardini M, van der Vliet A. Inhibition of neutrophil apoptosis by acrolein: a mechanism of tobacco-related lung disease? Am J Physiol Lung Cell Mol Physiol. 2001;281(3):L732–L739. doi: 10.1152/ajplung.2001.281.3.L732. [DOI] [PubMed] [Google Scholar]
- 14.Al Moamary MS. Tobacco consummation: is it still a dilemma? Ann Thorac Med. 2010;5(4):193. doi: 10.4103/1817-1737.69103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scherer G, Richter E. Biomonitoring exposure to environmental tobacco smoke (ETS): a critical reappraisal. Hum Exp Toxicol. 1997;16(8):449–459. doi: 10.1177/096032719701600806. [DOI] [PubMed] [Google Scholar]
- 16.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol. Rev. 1996;18(2), 188–204. [DOI] [PubMed]
- 17.Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, et al. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol. 2008;151(1):42–50. doi: 10.1111/j.1365-2249.2007.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall AL, Chetwynd A, Morris JA, Placzek M, Smith C, Olabi A, et al. Type 1 diabetes mellitus in childhood: a matched case control study in Lancashire and Cumbria, UK. Diabet Med. 2004;21(9):1035–1040. doi: 10.1111/j.1464-5491.2004.01282.x. [DOI] [PubMed] [Google Scholar]
- 19.McMillan SA, Douglas JP, Archbold GP, McCrum EE, Evans AE. Effect of low to moderate levels of smoking and alcohol consumption on serum immunoglobulin concentrations. J Clin Pathol. 1997;50(10):819–822. doi: 10.1136/jcp.50.10.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holt PG. Immune and inflammatory function in cigarette smokers. Thorax. 1987;42(4):241–249. doi: 10.1136/thx.42.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arinola OG, Akinosun OM, Olaniyi JA. Passive-and active-cigarette smoking: effects on the levels of antioxidant vitamins, immunoglobulin classes and acute phase reactants. Afr J Biotechnol. 2013;10(32):6130–6132. [Google Scholar]
- 22.Ussher M, West R, Evans P, Steptoe A, McEwen A, Clow A, et al. Acute reduction in secretory immunoglobulin A following smoking cessation. Psychoneuroendocrinology. 2004;29(10):1335–1340. doi: 10.1016/j.psyneuen.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 23.McSharry C, Banham SW, Boyd G. Effect of cigarette smoking on the antibody response to inhaled antigens and the prevalence of extrinsic allergic alveolitis among pigeon breeders. Clin Exp Allergy. 1985;15(5):487–494. doi: 10.1111/j.1365-2222.1985.tb02299.x. [DOI] [PubMed] [Google Scholar]
- 24.Aula FA, Qadir FA. Effects of cigarette smoking on some immunological and hematological parameters in male smokers in Erbil city. Jordan J Biol Sci. 2013;6(2):159–166. doi: 10.12816/0000274. [DOI] [Google Scholar]
- 25.Flouris AD, Poulianiti KP, Chorti MS, Jamurtas AZ, Kouretas D, Owolabi EO, et al. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem Toxicol. 2012;50(10):3600–3603. doi: 10.1016/j.fct.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Fernández JAF, Prats JM, Artero JVM, Mora AC, Fariñas AV, Espinal A, et al. Systemic inflammation in 222.841 healthy employed smokers and nonsmokers: white blood cell count and relationship to spirometry. Tob Induc Dis. 2012;10(1), 1–8. [DOI] [PMC free article] [PubMed]
- 27.Shenwai M, Aundhakar NV. Effect of cigarette smoking on various haematological parameters in young male smokers. Indian J Basic Appl Med Res. 2012;2(5):386–392. [Google Scholar]
- 28.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J. 2005;26(17):1765–1773. doi: 10.1093/eurheartj/ehi183. [DOI] [PubMed] [Google Scholar]
- 29.Zafar I, Mohammad KN, Nisar M, Rashida M, Assadullah BS, Mohammad SA. Effect of cigarette smoking on erythrocytes, leukocytes and haemoglobin. J Med Sci. 2003;3(3):245–250. doi: 10.3923/jms.2003.245.250. [DOI] [Google Scholar]
- 30.Schaberg T, Theilacker C, Nitschke OT, Lode H. Lymphocyte subsets in peripheral blood and smoking habits. Lung. 1997;175(6):387–394. doi: 10.1007/PL00007585. [DOI] [PubMed] [Google Scholar]
- 31.Freedman DS, Flanders WD, Barboriak JJ, Malarcher AM, Gates L. Cigarette smoking and leukocyte subpopulations in men. Ann Epidemiol. 1996;6(4):299–306. doi: 10.1016/S1047-2797(96)00024-5. [DOI] [PubMed] [Google Scholar]
- 32.Whitehead TP, Robinson D, Allaway SL, Hale AC. The effects of cigarette smoking and alcohol consumption on blood haemoglobin, erythrocytes and leucocytes: a dose related study on male subjects. Clin Lab Haematol. 1995;17(2):131–138. [PubMed] [Google Scholar]
- 33.Schwartz J, Weiss ST. Cigarette smoking and peripheral blood leukocyte differentials. Ann Epidemiol. 1994;4(3):236–242. doi: 10.1016/1047-2797(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 34.Tollerud DJ, Clark JW, Brown LM, Neuland CY, Mann DL, Pankiw-Trost LK, et al. The effects of cigarette smoking on T cell subsets. Am Rev Respir Dis. 1989;139(6):1446–1451. doi: 10.1164/ajrccm/139.6.1446. [DOI] [PubMed] [Google Scholar]
- 35.Ogawa Y, Imaki M, Yoshida YSM, Tanada S. An epidemiological study on association between TLC and neutrophil counts and risk factors of IHD by smoking status in Japanese factory workers. Appl Human Sci. 1998;17(6):239–247. doi: 10.2114/jpa.17.239. [DOI] [PubMed] [Google Scholar]
- 36.Nagai A, Ishihara Y, Takizawa T. Leukocyte number, protein-inhibitor, and complement in smokers. Nihon Kyobu Shikkan Gakkai zasshi. 1992;30(6):1050–1055. [PubMed] [Google Scholar]
- 37.Glader P, Möller S, Lilja J, Wieslander E, Löfdahl CG, von Wachenfeldt K. Cigarette smoke extract modulates respiratory defence mechanisms through effects on T-cells and airway epithelial cells. Respir Med. 2006;100(5):818–827. doi: 10.1016/j.rmed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164(20):2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 39.Kalra R, Singh SP, Savage SM, Finch GL, Sopori ML. Effects of cigarette smoke on immune response: chronic exposure to cigarette smoke impairs antigen-mediated signaling in T cells and depletes IP3-sensitive Ca2 + stores. J Pharm Exp Ther. 2000;293(1):166–171. [PubMed] [Google Scholar]
- 40.Geng Y, Savage SM, Razani-Boroujerdi S, Sopori ML. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T cell anergy. J Immunol. 1996;156(7):2384–2390. [PubMed] [Google Scholar]
- 41.Cohen S, Tyrrell DA, Russell MA, Jarvis MJ, Smith AP. Smoking, alcohol consumption, and susceptibility to the common cold. Am J Public Health. 1993;83(9):1277–1283. doi: 10.2105/AJPH.83.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mili F, Flanders WD, Boring JR, Annest JL, Destefano F. The associations of race, cigarette smoking, and smoking cessation to measures of the immune system in middle-aged men. Clin Immunol Immunopathol. 1991;59(2):187–200. doi: 10.1016/0090-1229(91)90017-5. [DOI] [PubMed] [Google Scholar]
- 43.Robbins CS, Franco F, Mouded M, Cernadas M, Shapiro SD. Cigarette smoke exposure impairs dendritic cell maturation and T cell proliferation in thoracic lymph nodes of mice. J Immunol. 2008;180(10):6623–6628. doi: 10.4049/jimmunol.180.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanigawa T, Araki S, Nakata A, Kitamura F, Yasumoto M, Sakurai S, et al. Increase in memory (CD4 + CD29 + and CD4 + CD45RO +) T and naive (CD4 + CD45RA +) T-cell subpopulations in smokers. Arch Environ Health. 1998;53(6):378–383. doi: 10.1080/00039899809605724. [DOI] [PubMed] [Google Scholar]
- 45.Moszczyński P, Żabiński Z, Rutowski J, Słowiński S, Tabarowski Z. Immunological findings in cigarette smokers. Toxicol Lett. 2001;118(3):121–127. doi: 10.1016/S0378-4274(00)00270-8. [DOI] [PubMed] [Google Scholar]
- 46.McAllister-Sistilli CG, Caggiula AR, Knopf S, Rose CA, Miller AL, Donny EC. The effects of nicotine on the immune system. Psychoneuroendocrinology. 1998;23(2):175–187. doi: 10.1016/S0306-4530(97)00080-2. [DOI] [PubMed] [Google Scholar]
- 47.Sopori ML, Gairola CC, DeLucia AJ, Bryant LR, Cherian S. Immune responsiveness of monkeys exposed chronically to cigarette smoke. Clin Immunol Immunopathol. 1985;36(3):338–344. doi: 10.1016/0090-1229(85)90054-6. [DOI] [PubMed] [Google Scholar]
- 48.Asthana A, Johnson HM, Piper ME, Fiore MC, Baker TB, Stein JH. Effects of smoking intensity and cessation on inflammatory markers in a large cohort of active smokers. Am Heart J. 2010;160(3):458–463. doi: 10.1016/j.ahj.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Islam MM, Amin MR, Begum S, Akther D, Rahman A. Total count of white blood cells in adult male smokers. J Bangladesh Soc Physiol. 2007;2:49–53. doi: 10.3329/jbsp.v2i0.985. [DOI] [Google Scholar]


