Abstract
Coelomic fluid of Lumbricus rubellus (CFL) has attracted interest due to its pharmacological properties, including antitumor effect. Furthermore, it is necessary to evaluate the response to treatment with new cancer therapeutic agents. This study aims to investigate whether the combination of CFL and 5-fluorouracil could reduce FAK protein level and iCa2+ and enhance p21 level. Furthermore, it is necessary to evaluate the response to treatment with new cancer therapeutic agents. After 24 hours of treatment, it was necessary to assess the percentage of apoptosis, FAK, and p21 protein expression by flow cytometry. iCa2+ concentration was measured using immunofluorescence. The combination therapy of CFL with 5-fluorouracil potently suppressed six treatment groups were included in this study. HT-29 cell lines were cultured and divided into six groups: group 1 was treated with vehicle (negative control), groups 2-5 were treated with 5-fluorouracil, groups 3-5 were treated with either CFL 5, 10, or 20 µg/ml immediately after 5-fluorouracil, and group 6 was treated with CFL 20 µg/ml, the progression of colorectal cancer. Combination of CFL and 5-fluorouracil significantly decreased FAK expression (p<0.05), iCa2+ (p<0.05), and increased p21 expression (p<0.05) in HT-29 cells. Our results suggest that CFL has an anticancer potential in colorectal cancer when combined with 5-fluorouracil.
1. Introduction
Cancer is one of the leading causes of death worldwide. Colorectal cancer is one of the most common malignancies with a high prevalence and low 5-year survival rate [1]. The incidence of colorectal cancer as well as the mortality rate is very high. Chemotherapy allows for the treatment of patients with advanced-stage tumors or those who have relapsed. 5-Fluorouracil (5-FU) is a cytostatic drug used for the treatment of various types of cancers especially colon cancer [2]. An antimetabolite chemotherapy agent such as 5-FU has a primary function to convert thymidylate synthetase (TS) into deoxythymidine monophosphate (dTMP) in cells that are actively proliferating [3]. However, due to the high intrinsic resistance of colon cancer to currently available agents, 5-FU alone and 5-FU-based combination chemotherapy are not expected to achieve great success [3, 4]. Therefore, new therapeutic strategies are urgently needed. Currently, combination chemotherapy and adjuvant chemotherapy have achieved great success in the treatment of malignant disease. A combination of conventional chemotherapies with new therapies directly targeted against the molecular changes in colon cancer seems to be the most promising strategy.
Focal adhesion kinase (FAK) has appeared to be firmly identified to and take a crucial part of cancers growth. FAK expression was elevated in an assortment of tumors metastatic [5, 6]. In addition, the balance of FAK expression may affect the ability of growth of tumor cells, proliferation, and a balance of the particular FAK inhibitors with cytotoxic may be a promising anticancer treatment [7, 8].
Lumbricus rubellus, an earthworm species of the class Oligochaeta, belongs to the family Lumbricidae. It is widely found throughout the mainland Europe and also worldwide, including Indonesia. Bioactive compounds have been extracted from earthworms in various countries and used as therapeutic materials for thousands of years [9]. Some studies have shown that they have therapeutic properties, such as anti-inflammatory, antioxidant, antitumor, and antibacterial [9, 10]. The therapeutic properties are attributable to their coelomic fluid (CFL), which contains biological substances such as lectin, polysaccharides, proteases, antibacterial peptides, and fibrinolytic enzymes [10].
Their CFL was found to have a cytotoxic effect in HeLa cells after exposure for 48 hours [11]. Antitumor effects also were showed by CFL and inhibited the proliferation of HeLa cells [9–11]. The CFL of Eudrilus eugeniae also had a cytotoxic effect on HeLa cells, leukemia cells, and brain tumor cells [12]. In colorectal cancer progression, FAK plays a critical role. It is an oncogenic protein located in the cytoplasm and regulates cell attachment, proliferation, survival, and motility [13]. The activation of FAK also leads to increased expression of cyclin D1 [14]. Integrin-dependent and independent FAK are localized to the nucleus and interact directly with p53 to promote cell proliferation and survival through p53 degradation [15]. A protein downstream of p53 protein such as p21 plays a role in regulating proliferation, apoptosis, and metastasis of cancer cells. Activation of p21 is expected to suppress the progression of colon cancer [16]. Recently, increased Bcl-2 expression was reported to increase intracellular Ca2+ (iCa2+) in the cytoplasm [17]. Alterations of iCa2+ in cancer cells play essential roles in tumor initiation, angiogenesis, progression, and metastasis [18]. The increase in iCa2+ appeared to be mediated by a calcium release mechanism. Furthermore, targeting [iCa2+] signaling may be a novel strategy to develop more effective treatments for colon cancer. It is signaling of [iCa2+] is critical for regulating cell migration and invasion. However, whether it has antitumor activity against colon cancer is not yet clear.
This study showed evidence that the CFL of L. rubellus has antitumor properties in vitro. On the basis of the antitumor effect of CFL, we examined the therapeutic potential of the CFL in the inhibition of colorectal cancer. Further analysis revealed that p21 activated by this fluid was associated with reduced FAK and [iCa2+].
2. Materials and Methods
2.1. Collection of Earthworms
The earthworms were collected from a commercial vermin-culture, CV. RAJ Organic-Malang-Indonesia, and maintained in plastic tubs containing decomposed organic matter at Department of Parasitology, Faculty of Medicine, Brawijaya University. The experiments were performed in accordance with the guidelines and approval (No.238/EC/KEPK/10/2017) of the Institutional Animal Care and Use Committee of Brawijaya University and followed institutional requirements concerning the care and handling of animals according to Guiding Principles for the Care and Use of Animals for Scientific Purposes in the Institutional Animal Care and Use Committee (IACUC).
2.2. Coelomic Fluid Collection by Heat and Cold Shock Method
The earthworms were washed in distilled water and then placed in a glass funnel. Worms (20 grams) were kept immersed under 25 ml of warm water (45-50°C) in a glass beaker. This heat shocks method yielded 0.5 ±0.25 ml of coelomic fluid. Heat shock was used as well as in a similar way ice cubes in the cold shock method. All these fluids were subsequently employed for further experimentation. The treatment was alternated at a gap of five minutes to overcome the shock effect. The fluid released was collected into the tubes.
2.3. Coelomic Fluid Precipitation
Coelomic fluid was precipitated by Ammonium sulfate and was slowly added to the coelomic fluid to make a final concentration. The pellet collected by centrifugation was resuspended in 20 mM Tris-HCl (pH 8.0) followed by centrifugation. Then, the pellet was resuspended in cold acetone by adding the acetone slowly, followed by 20-minute incubation. The remaining acetone was removed from the pellet by centrifugation. Protein concentrations were measured using the Bradford method with bovine serum albumin (BSA) used as the standard.
2.4. Maintenance and Culture of HT-29 Cells
The cell line was purchased from ATTC, USA. Human colon cancer cell line HT-29 was maintained in McCoy medium supplemented with 10% FBS (Fetal Bovine Serum) FCS, 2 mM glutamine, 100 IU/mL penicillin, and 0,1 mg/mL streptomycin. The cells were grown in the humidified CO2 incubator (Binder's, USA) at 5% CO2 level at 37°C. Passage of cells was carried out by 0.05% trypsin-EDTA at 70-80% confluence. Cell line HT-29 was cultured at concentration 5x105 cells/ ml at 24-well plate, until confluent state reached 80%. The cells were cryopreserved with DMSO and stored at -80°C for further stocks.
2.5. Experimental Groups and Treatments
HT-29 cells were treated with CFL and 5-fluorouracil for 24 hours in different doses. HT-29 cells were cultured and divided into six groups: group 1 was control, groups 2-5 were treated with 5-fluorouracil, groups 3-5 were treated with either CFL 5, 10, or 20 µg/ml immediately after 5-fluorouracil, and group 6 was treated with CFL 20 µg/ml.
2.6. Apoptosis Analysis
Apoptosis cells were determined quantitatively by flow cytometry using the annexin V-conjugated FITC (eBioscience) following the manufacturer's protocol. Briefly, HT-29 cells were treated with 5-fluorouracil alone or with CFL for 24 hours, and cells were harvested, washed with PBS, and incubated with annexin V (FITC) and Propidium Iodide for cell staining in binding buffer (room temperature, 15 minutes in the dark). The stained cells were analyzed using Cell Quest software.
2.7. Flow Cytometry Analysis for FAK and p21
FAK and p21 percentages were analyzed by flow cytometry according to Zuryn et al. (2014) [19]. Briefly, cells grown in 6-well plates were harvested, washed with PBS, centrifuged (5 minutes, 300 × g), and fixed with 1% methanol-free, ultrapure formaldehyde. After incubation on ice for 15 minutes, cells were centrifuge for 15 minutes, 300 x g). The cells pellets were permeabilized by the addition of 1 ml of ice-cold 50% (v/v) methanol. After that, cells were incubated for 30 minutes on ice, washed twice with cold PBS, and resuspended in 0.5% bovine serum albumin (BSA; Sigma-Aldrich) for 10 minutes. For intracellular staining, the cell suspensions were transferred into flow cytometric tubes containing 20 μl of FITC conjugated monoclonal anti-human p21 or FAK (Santa Cruz Biotechnology, Inc.), and 200 μl of 0.5% BSA. Following a 45-minute incubation (4°C, in the dark) and washing with PBS, the cells were centrifuged (5 minutes, 500 × g) to wash off excess antibody and resuspended in 200 μl of PBS for flow cytometric analysis on FACS Calibur (Becton-Dickinson). Cell Quest software (Becton-Dickinson) was used to calculate the percentage of p21 or FAK positive cells.
2.8. Measurement of Intracellular Calcium Using Fluorescence Microscopy
Measurement of intracellular calcium accumulation Fluo 3-AM (Bioscience, USA), a fluorescent probe, was applied to determine intracellular calcium. Briefly, cells were washed twice with PBS at 800 g for 10 minutes each and resuspended in McCoy medium at 1×105 cells/ml. The cell suspension was plated on polylysine coated slides and allowed to adhere for 10 minutes. HT-29 cell were loaded with BSA 1% and incubated for 60 minutes at room temperature. After loading BSA 1%, 10 µM fluo-3/AM was added and incubated for 60 minutes at 37°C. After the incubation, samples were fixed for 60 minutes with 4% PFA and washed twice with PBS for 5 minutes each time. Fluo-3/AM were observed with 515nm wave and the Ca2+ were analyzed with imageJ software.
2.9. Statistical Analyses
Data are expressed as mean ± SEM and statistically evaluated with one-way ANOVA followed by the Bonferroni post hoc test. Results were expressed as means ± standard deviation. Differences between means were determined by the Mann-Whitney U test (with the level of significance established at p<0.05). Statistical analysis was performed with SPSS software version 20 (IBM Corporation, USA).
3. Results
3.1. CFL in Combination with 5-FU Induced Apoptosis of HT-29 Cells
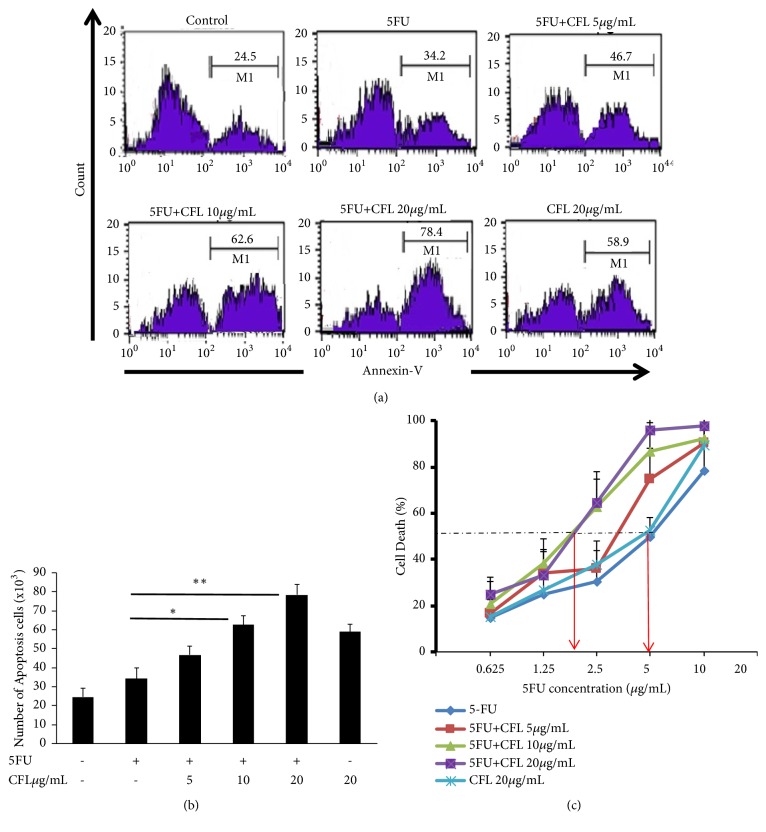
The combination of CFL and 5-FU exerts an enhanced apoptosis effect on HT-29 cells. As for confirming the improved apoptosis effect of the cotreatment, we assessed the number of apoptosis of HT-29 cells (Figure 1). This study had definite effect in apoptosis after treatment with 5-FU alone or in combinations with f 5, 10, and 20 μg/mL CFL. After 24 hours of treatments, the apoptosis rate significantly increased by half or more (p<0.001). Treatment with 5 μg/mL 5-FU only slightly increased the number of apoptosis cells (p<0.05)
Figure 1.
The combination therapy of CFL with 5-fluorouracil induced apoptosis in HT-29 cell line. (a) HT-29 cell line was cultured and divided into six groups: group 1 was without treatment (control); group 2 was treated with 5-fluorouracil, groups 3-5 were treated with either CFL 5, 10, or 20 µg/ml immediately after 5- fluorouracil, and group 6 was treated with CFL 20 µg/ml. The number of apoptosis cells showed that combination treatment has higher number than single treatment group, respectively (p<0.05; p<0.05; p<0.05). Red lines (isotype control). (b) Number of annexin-V positive cells indicated apoptosis cells. (c). Dose response of CFL showed cytotoxic synergistic effects (results shown are mean ± SD, with n =4 ∗p < 0.05, ∗∗p < 0.001.
3.2. Reduced FAK Expression Induced by CFL Combined with 5-FU
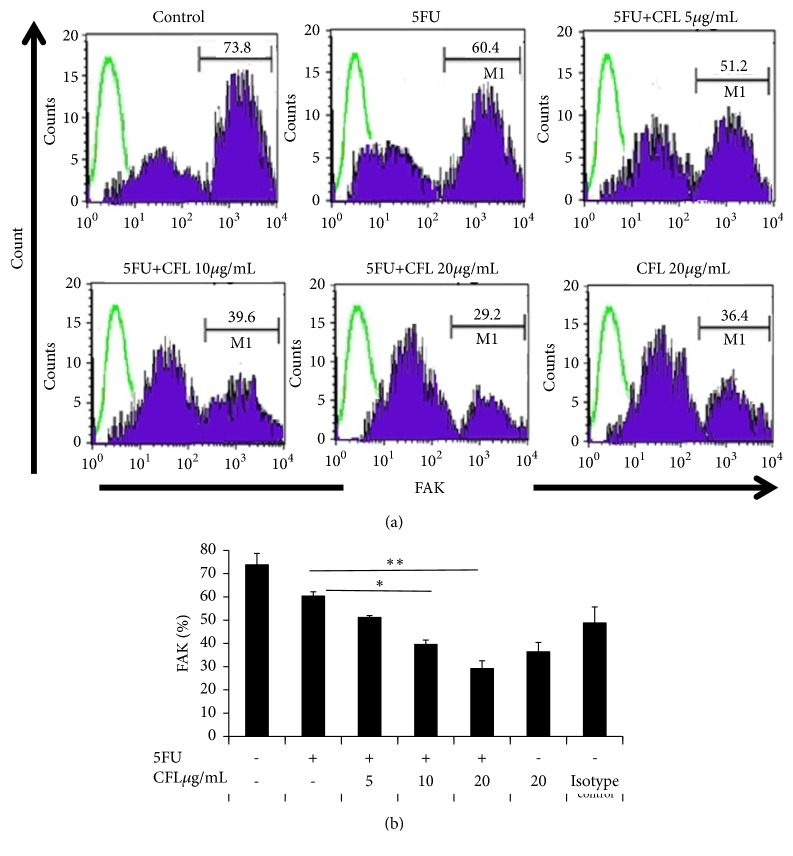
As to confirm the enhanced anticancer effect of the cotreatment, we assessed the expression level of FAK. FAK expression was determined by using flow cytometry. As shown in Figure 2, CFL alone decreased the percentage of FAK expression. The combination of 5-FU and CFL exert an enhanced anticancer effect on HT-29 cells. The representative images shown in Figure 2 demonstrated a clear decreased FAK expression after treatment with CFL alone or in combinations with CFL and 5-FU. After 24 hours of treatments, the FAK expressions significantly decreased by half (p<0.05). Treatment with 5-FU only slightly reduced the expression of FAK (p<0.05). When the combination of CFL and 5-FU was applied, the percentage of FAK expression was lower than 5-FU or CFL alone (p<0.05), which indicated that FAK expression was decreased via CFL and 5-FU combinations (Figure 2).
Figure 2.
The combination therapy of CFL with 5-fluorouracil reduced the percentage of FAK protein level in HT-29 cells. HT-29 cells were cultured and divided into six groups: group 1 was without treatment (control); group 2 was treated with 5-fluorouracil (5FU), groups 3-5 were treated with either CFL 5, 10, or 20 µg/ml added immediately after 5-fluorouracil, and group 6 was treated with CFL 20 µg/ml. The percentage of FAK of 5FU group was significantly different from the three groups of 10 µg/mL (p<0.05) and 20 µg/mL (p<0.05) and CFL groups (p<0.05). (a) Results are representative of four independent experiments. Green lines show isotype control IgG2b. (b) Number of FAK positive cells. Results shown are mean ± SD, with n =4 replicates in each group. ∗p<0.05, ∗∗p < 0.001.
3.3. Reduced iCa2+ Expression Induced by CFL Combined with 5-FU
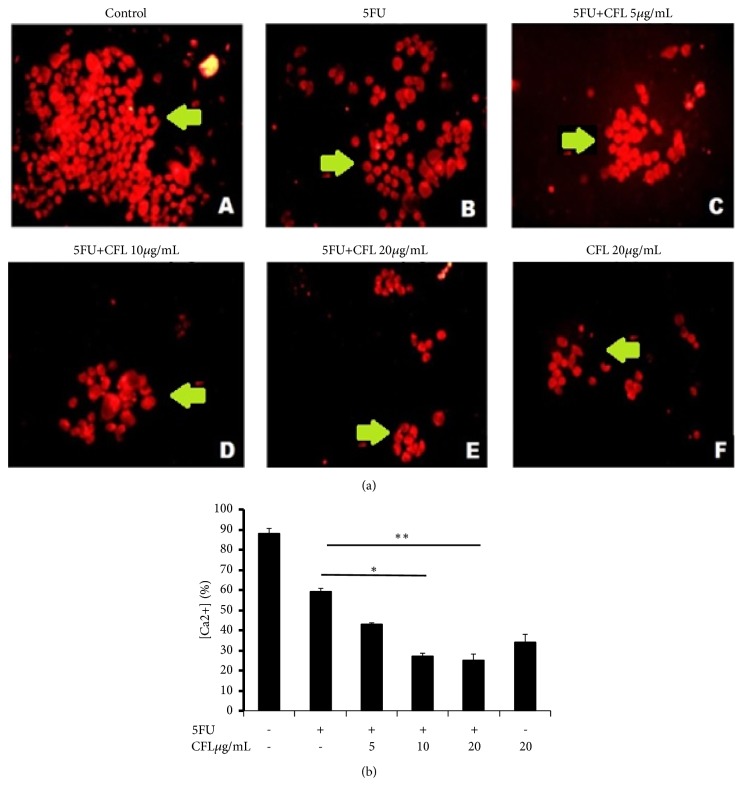
Since the administration of CFL and 5-FU combinations caused change in expression of FAK protein, we hypothesized that CFL and 5-FU combinations could have effects on the decrease of iCa2+ concentration. We analyzed the effect of CFL and 5-FU combinations in iCa2+ concentration using immunofluorescence. As shown in Figure 3, the number of iCa2+ expression in control group was significantly increased (p<0.05). The treatment of CFL with or without 5-FU combinations can decrease significantly iCa2+ concentration compared with 5-FU alone (p<0.05). In this study, based on Figure 3 combination of 10 μg/ml CFL and 5 μg/ml 5-FU was able to decrease the percentage of FAK compared with 5-FU only.
Figure 3.
The combination therapy of CFL with 5-fluorouracil reduced the expressions of Ca2+ intracellular level in HT-29 cells. (A) Control group. (B) Group was treated with 5FU only. (C), (D), and (E) HT-29 cells were treated with CFL 5, 10, and 20 μg/mL, respectively, after 5-fluorouracil, (F) HT-29 cells were treated with CFL only. Green arrow: Ca2+ intracellular positive cell. All images were magnified at representative 4 independent experiments (G). Number of Ca2+ intracellular positive cells. The result of [iCa2+] expression also showed that combination groups have lower expression than 5FU only group, respectively (p<0.05; p<0.05; p<0.05). ∗p-value < 0.05; ∗∗p-value < 0.001.
3.4. Combination of 5-FU and CFL Increased p21 Presentation in HT-29-Cell Line
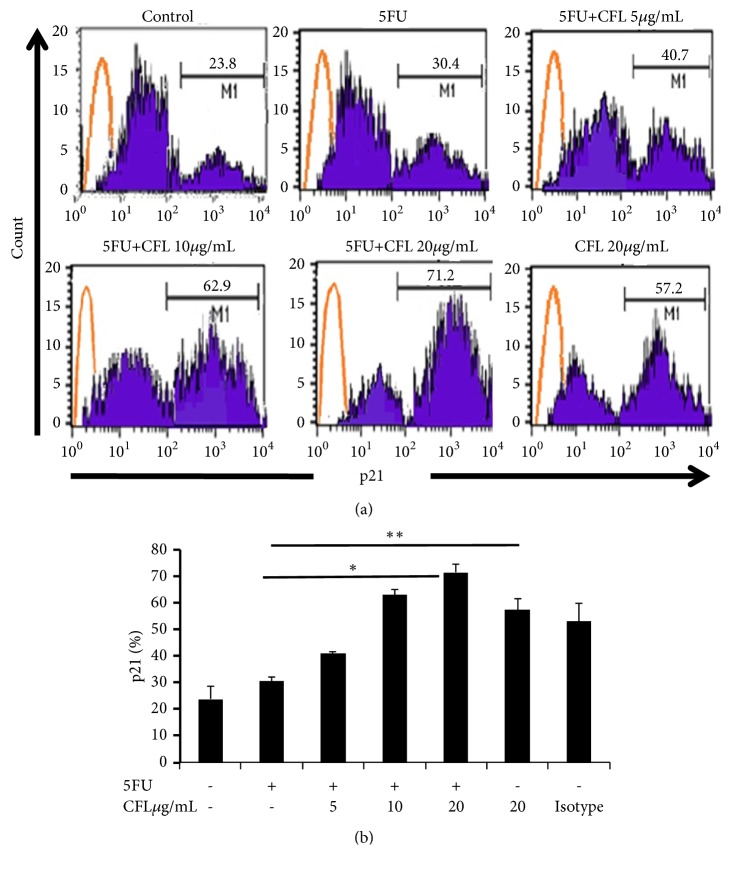
The effect of CFL on HT-29 cells treated with combination therapy: p21 expression was determined by using flow cytometry. The percentage of p21 for each group was significantly increased (p< 0.05). The percentage of p21 between control and treatment groups was significantly increased (p<0.05) and CFL (p<0.05) (Figure 4). In this study, CFL level significantly influenced the increasing of p21.
Figure 4.
CFL combined with 5-FU reduced p21 expressions of HT-29. The expression of p21 was assessed to evaluate the antimetastatic effect of CFL combined with 5-FU. (a) The percentage of p21 positive cells was analyzed using a FACS Calibur flow cytometer (BD Biosciences). Numbers represent the percentages of p21 positive cells were shown, orange lines (isotype control IgG2b). Results are representative of four independent experiments. (b) The percentage of p21 positive cells was shown inside the panels. Results shown are mean+SD, with n=4 replicates in each group. ∗p-value < 0.05; ∗∗p-value p< 0.001.
4. Discussion
In this study, we investigated the enhanced antimetastatic effect of 5-FU on HT-29 cells when combined with CFL. The results showed that calcium-mediated FAK protein is involved in the reduction of p21 signaling. Treatment of HT-29 cells with the combination of 5-FU and CFL reduced FAK expression and [iCa2+] levels and showed antitumor ability through activation of p21 protein. Previously, some proteins and peptides with antitumor activities have been found in CFL [20–22].
[iCa2+] regulates FAK protein signaling, which is involved in cancer cell survival signaling. [iCa2+] levels in cancer cells are affected by changes in Ca2+ transporter, channel, and pump [21]. Changes in the expression or activity of Ca2+ channels and pumps could promote role in cancer [23].
In cancer, the antiapoptotic protein Bcl-2 is commonly deregulated and appears to modulate IP3K. The best characterised Ca2+ store is the ER which houses IP3- and ryanodine-sensitive Ca2+ channels [21–23]. Moreover, Bcl-2 can decrease the sensitivity of the mitochondrial Ca2+ uptake process as well as reducing the ability of Ca2+ to induce apoptosis [24–26]. The influx of Ca2+ through plasma membrane channels, such as calcium release-activated calcium channel protein 1 (ORAI1) and transient receptor potential subfamily V member 6 (TRPV6) cation channel, results in increased Ca2+ entry and induces cell proliferation [24]. Our results suggest that the two pathways activated by CFL induced p21 activation; subsequently, a PI3K pathway leads to the sensitization of (transient receptor potential subfamily V member 1 (TRPV1) cation channel. This sensitization increased [iCa2+] via voltage-gated channels [24, 25]. In the same way, the increase in [iCa2+] will activate calpain, which degrades FAK and enhances the motility of cancer cells [25]. Calpain is a cysteine protease which plays a critical role in focal adhesion dynamics in motile cells [26].
In malignant cells, iCa2+ regulates the molecular machinery of cell migration. FAK plays a key role in apoptosis and migration of cancer cells [27]. FAK inhibitors have been shown to reduce tumor metastasis. Due to the role of FAK in EMT, calpain-dependent proteolysis of FAK may have the potential to suppress cancer cell motility [26, 27]. Activated FAK plays an important role as a key signal mediator in tumor progression and metastasis. The increasing of FAK expression contributes to metastasis and tumor proliferation [27, 28].
These results suggest that by increasing [iCa2+] and activation of IP3, the efficacy of the combination of CFL with 5-FU could be increased resulting in a higher rate of apoptosis. Tumorigenesis is associated with the inactivation of p21, a protein downstream from p53 that regulates metastasis.
Several promising colon cancer studies revealed that FAK is associated with p21. FAK has been shown to localize in the nucleus and interact directly with p53 to promote cell proliferation and survival through p53 degradation by mdm2 [28]. This result suggested that the combination of CFL and 5-FU could increase p21 expression through the inhibition of FAK protein. The inhibition of FAK protein led to increase in p21 expression through p53 pathway [29].
The phosphorylation of FAK such as [iCa2+] at its serine residues, as shown for HT-29 cells with [iCa2+], is activated and leads to the activation of FAK by phosphorylating FAK. This study showed that a different stimulus was able to increase p21 levels irrespective of FAK status. Thus, FAK functions in these advanced cancer cells were to suppress p53-dependent transcription of p21 by degradation. In addition, we found that FAK interacts with several binding partners, such as p53 and p21 [10, 29], FAKs link to apoptotic pathways and that p21 inhibits FAK transcription in vitro. There was an interaction between p53 and FAK as an example of focal adhesion protein functioning in the signaling between extracellular matrix and nucleus. FAK can also block p53 transcriptional activity of p21 [10, 30, 31]. Thus, this study suggested that p21 can induced apoptosis through downregulation of metastasis-related genes, such as FAK. We have shown recently that p21 can downregulate FAK expression inhuman cancer cells [28, 31]. HT-29 cells express a p53 mutated form, which can still induce p21 and cell cycle arrest. It was therefore somewhat surprising when several tumor-derived mutants of p53 were demonstrated to interact with both p63 and p73 [32, 33]. This interaction has been invoked to explain many of the gain-of-function effects of mutant p53. The mutant of p53 can bind to p63 and inhibit p63-transcriptional activity [34]. This report explains that direct interaction of mutant p53 with p63 was able to transactivate p21. Then, we demonstrated a high correlation between FAK overexpression and p21 in breast cancer cells.
Our results showed that a decrease in the percentage of FAK was related to the dose of CFL. This result proves that CFL administration was able to reduce FAK protein expression. This further suggests that 5-FU as a single therapeutic agent has not been sufficient for colon cancer treatment. The increase in p21 levels was related to the CFL dose, proving that CFL treatment can affect p21 protein levels.
Ca2+ increase in cancer cells is affected by changes in the Ca2+ transporter, channel, and pump [25, 32]. Ca2+ concentrations can be used as a therapeutic target and inhibiting Ca2+ transporter, channel, and pump would regulate Ca2+ levels [35, 36]. The results indicated a strong correlation between FAK and Ca2+. According to Sundaramoorthy et al. [37], it was revealed that increased intracellular calcium in cancer cells activates the cysteine protease of calpain and induces the cleavage and transfer of FAK into the nucleus [35, 37]. In colorectal cancer, Bcl-2 prevents apoptosis by suppressing p53 [32–34]. p53 activates Kruppel like factor-4 (KLF4) transcription thereby causing a decrease in the level of transcriptional factor Cyclin-dependent kinase inhibitor 1A (CDKN1A), which is a p21 protein-coding gene; eventually, p21 levels decrease [37–40]. CCF-1 acting as an analog of TNF is expected to suppress Bcl-2 thus increasing the expression of p21 and suppressing FAK and iCa2+. Based on this finding, Bcl-2 plays a significant role in decreasing p21 and activating FAK and iCa2+. Furthermore, by inhibiting Bcl-2 in the coelomic fluid using TNF analogs [39–42], activation of FAK and iCa2+ can be suppressed.
5. Conclusions
Our results suggest that FAK can be a new therapeutic target for colon cancer, and the development of selective FAK inhibitors may be a promising way to enhance CFL chemosensitivity in colon cancer.
Acknowledgments
We would like to acknowledge Directorate General of Higher Education, Ministry of National Education, and Culture of Republic Indonesia, for the provided grant for this research. We gratefully thank Heni Endrawati.SSi, Wahyudha Ngatiril Lady, S.Si, Suci Megasari, S.Si, M.P, Ami Maghfironi, S.Si, and Bunga Prihardina, S.Si, for technical assistance.
Abbreviations
- 5-FU:
5-Fuoro Uracil
- Bcl-2:
B-cell lymphoma 2
- CCF-1:
Coelomic Cytolytic Factor-1
- CDKN1A:
Cyclin-dependent kinase inhibitor 1A
- CFL:
Coelomic fluid Lumbricus
- dTMP:
Deoxythymidine monophosphate
- FAK:
Focal adhesion kinase
- IP3:
Inositol 1,4,5-trisphosphate
- KLF4:
KLF4 Kruppel like factor-4
- ORAI1:
Calcium release-activated calcium channel protein 1
- TNF:
Tumor Necrosis Factor
- TRPV1:
Transient receptor potential cation channel subfamily V member 1
- TRPV6:
Transient receptor potential cation channel subfamily V member 6
- TS:
Thymidylate synthetase.
Data Availability
These data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest with regard to the publication of this paper.
References
- 1.Ng S. C., Wong S. H. Colorectal cancer screening in Asia. British Medical Bulletin. 2013;105(6):29–42. doi: 10.1093/bmb/lds040. [DOI] [PubMed] [Google Scholar]
- 2.Abdullah M., Sudoyo A. W., Utomo A. R., Fauzi A., Rani A. A. Molecular profile of colorectal cancer in Indonesia: is there another pathway? Gastroenterology and Hepatology from Bed to Bench. 2012;5(2):71–78. [PMC free article] [PubMed] [Google Scholar]
- 3.Hu T., Li Z., Gao C., Cho C. H. Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World Journal of Gastroenterology. 2016;22(30):6876–6889. doi: 10.3748/wjg.v22.i30.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedbrant A., Erlandsson A., Delbro D., Wijkander J. Conditioned media from human macrophages of M1 phenotype attenuate the cytotoxic effect of 5-fluorouracil on the HT-29 colon cancer cell line. International Journal of Oncology. 2015;46(1):37–46. doi: 10.3892/ijo.2014.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon H., Dehart J. P., Murphy J. M., et al. Understanding the Roles of FAK in Cancer. Journal of Histochemistry & Cytochemistry. 2015;63(2):114–128. doi: 10.1369/0022155414561498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim S. T., Chen X. L., Lim Y., et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Molecular Cell. 2008;29(1):9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serrels A., McLeod K., Canel M. The role of FAK's kinase domain in the proliferation and migration of squamous cell carcinoma cells. International Journal of Cancer. 2012;131(2):287–297. doi: 10.1002/ijc.26351. [DOI] [PubMed] [Google Scholar]
- 8.Heffler M., M. Golubovskaya V., Conroy J., et al. FAK and HAS inhibition synergistically decrease colon cancer cell viability and affect expression of critical genes. Anti-Cancer Agents in Medicinal Chemistry. 2013;13(4):584–594. doi: 10.2174/1871520611313040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C., Chen M., Li X., Yang M., Wang Y., Yang X. Purification and function of two analgesic and anti-inflammatory peptides from coelomic fluid of the earthworm, Eisenia foetida. Peptides. 2017;89:71–81. doi: 10.1016/j.peptides.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Endharti A. T., Baskoro A. D., Norahmawati E. Therapeutic effect of soluble worm protein acting as immune regulatory on colitis. Asian Pacific Journal of Tropical Biomedicine. 2017;7(1):70–77. doi: 10.1016/j.apjtb.2016.11.006. [DOI] [Google Scholar]
- 11.Hua Z., Wang Y-H., H-W Cao. Purification of a protein from CFL of the earthworm Eisenia foetida and evaluation of its hemolytic, antibacterial, and antitumor activities. Pharmaceutical Biology. 2011;49(3):269–275. doi: 10.3109/13880209.2010.508498. [DOI] [PubMed] [Google Scholar]
- 12.Dinesh M. S., Sridhar S., Chandana P. G., et al. Anticancer potentials of peptides of CFL of earthworm Eudrilus eugeniae. Biosciences Biotechnology Research Asia. 2013;10(2):601–606. [Google Scholar]
- 13.Ashton G. H., Morton J. P., Myant K., et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Developmental Cell. 2010;19(2):259–269. doi: 10.1016/j.devcel.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim S.-T., Chen X. L., Lim Y., et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Molecular Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kathryn G., Kim M., Owen J. FAK deletion promotes p53-mediated induction of p21, dna-damage responses and radio-resistance in advanced squamous cancer cells. PLoSOne. 2011;6(12) doi: 10.1371/journal.pone.0027806.e27806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L., Guo H., Zhan Y., et al. Specific up-regulation of p21 by a small active RNA sequence suppresses human colorectal cancer growth. Oncotarget. 2017;8(15):25055–25065. doi: 10.18632/oncotarget.15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu L., Shi W., Deshmukh R. R., Long J., et al. Tumor necrosis factor-a sensitizes breast cancer cells to natural products with proteasome-inhibitory activity leading to apoptosis. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0113783.e113783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart T. A., Yapa KTDS, Monteith G. R. Altered calcium signaling in cancer cells. Biochimicaet et Biophysica Acta. 2015;1848(10):2502–2511. doi: 10.1016/j.bbamem.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Dominic A., Roopa S., Jayaraman A., et al. In vitro antiproliferative effect of earthworm coelomic fluid of eudrilus eugeniae, eisenia foetida, and perionyx excavatus on squamous cell carcinoma-9 cell line: a pilot study. Pharmacognosy Research. 2017;9(suppl 1):S61–S66. doi: 10.4103/pr.pr_52_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Żuryń A., Litwiniec A., Gagat M., Drzewucka J., Gackowska L., Grzanka A. The influence of arsenic trioxide on the cell cycle, apoptosis and expression of cyclin D1 in the Jurkat cell line. Acta Histochemica. 2014;116(8):1350–1358. doi: 10.1016/j.acthis.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Mahendra K. V., Francies X., Yogendra K. V. Evaluation of cytotoxic and anti-tumor activity of partially purified serine protease isolate from the Indian earthworm Pheretima posthum. Asian Pacific Journal of Tropical Biomedicine. 2013;3(11):896–901. doi: 10.1016/S2221-1691(13)60175-6. [DOI] [Google Scholar]
- 22.Qu B., Al-Ansary D., Kummerow C., Hoth M., Schwarz E. C. ORAI-mediated calcium influx in T cell proliferation, apoptosis and tolerance. Cell Calcium. 2011;50(3):261–269. doi: 10.1016/j.ceca.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Cui C., Merritt R., Fu L., Pan Z. Targeting calcium signaling in cancer therapy. Acta Pharmaceutica Sinica B (APSB) 2017;7(1):3–17. doi: 10.1016/j.apsb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prevarskaya N., Skryma R., Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nature Reviews Cancer. 2011;11(8):609–618. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 25.Pan Z., Avila A., Gollahon L. Paclitaxel induces apoptosis in breast cancer cells through calcium--regulating mechanisms depending on external calcium conditions. International Journal of Molecular Sciences. 2014;15:2672–2694. doi: 10.3390/ijms15022672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerstein P. C., Patel K. M., Gomez T. M. Calpain-mediated proteolysis of talin and FAK regulates adhesion dynamics necessary for axon guidance. The Journal of Neuroscience. 2017;37(6):1568–1580. doi: 10.1523/JNEUROSCI.2769-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdulkader A., Wakkas F., John H., et al. Nuclear expression of phosphorylated focal adhesion kinase is associated with poor prognosis in human colorectal cancer. Anticancer Research. 2014;34(8):3969–3974. [PubMed] [Google Scholar]
- 28.Kyle P. F., Clare M. A., Ramkrishna M., et al. Mdm2 Is required for survival and growth of p53-deficient cancer cells. Cancer Research. 2017;77(14):1–11. doi: 10.1158/0008-5472.CAN-17-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim E. M., Jung C.-H., Kim J., Hwang S.-G., Park J. K., Um H.-D. The p53/p21 complex regulates cancer cell invasion and apoptosis by targeting Bcl-2 family proteins. Cancer Research. 2017;77(11):3092–3100. doi: 10.1158/0008-5472.CAN-16-2098. [DOI] [PubMed] [Google Scholar]
- 30.Endharti A. T., Permana S. T-Bet Is Dependent on Stat-4 Inhibiting Acute Colitis but Not Stat-1 Using L4 Somatic Antigen of Heligmosomoides polygyrus. The Scientific World Journal. 2018;2018:9. doi: 10.1155/2018/8571920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endharti A. T., Permana S. Extract from mango mistletoes Dendrophthoe pentandra ameliorates TNBS-induced colitis by regulating CD4+ T cells in mesenteric lymph nodes. BMC Complementary and Alternative Medicine. 2017;17(1):p. 468. doi: 10.1186/s12906-017-1973-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin H. M., Lee Y. J., Li G. Bcl-2 induces cyclin D1 promoter activity in human breast epithelial cells independent of cell anchorage. Cell Death & Differentiation. 2001;8(1):44–50. doi: 10.1038/sj.cdd.4400770. [DOI] [PubMed] [Google Scholar]
- 33.Chen L., Iraci N., Gherardi S., et al. p53 is a direct transcriptional target of MYCN in neuroblastoma. Cancer Research. 2010;70(1):1377–1388. doi: 10.1158/0008-5472.CAN-09-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strano S., Fontemaggi G., Costanzo A., et al. Physical interaction with human tumor-derived p53 mutants inhibits p63 activities. The Journal of Biological Chemistry. 2002;277(21):18817–18826. doi: 10.1074/jbc.M201405200. [DOI] [PubMed] [Google Scholar]
- 35.Petitjean A., Ruptier C., Tribollet V., Hautefeuille A., Chardon F., Cavard C., et al. Properties of the six isoforms of p63: p53-like regulation in response to genotoxic stress and cross talk with DeltaNp73? Carcinogenesis. 2008;29:273–281. doi: 10.1093/carcin/bgm258. [DOI] [PubMed] [Google Scholar]
- 36.Dötsch V., Bernassola F., Coutandin D., Candi E., Melino G. p63 and p73, the ancestors of p53. Cold Spring Harbor Perspectives in Biology. 2010;9 doi: 10.1101/cshperspect.a004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang M., Milner J. Bcl-2 constitutively suppresses p53-dependent apoptosis in colorectal cancer cells service. Genes Dev. 2003;17(1):832–837. doi: 10.1101/gad.252603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sundaramoorthy P., Sim J. J., Jang Y-S., et al. Modulation of intracellular calcium levels by calcium lactate affects colon cancer cell motility through calcium-dependent calpain. PLoS ONE. 2015;10(1) doi: 10.1371/journal.pone.0116984.e0116984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Permana S., Sholihah Z., Pearlindah., et al. Cytotoxic effects and anti-proliferative cancer activity of coelomic fluid from Lumbricus rubellus promotes apoptosis and reduces G2/M phase progression in HT-29 cells. Journal of Applied Pharmaceutical Science. 2018;8(11):28–34. [Google Scholar]
- 40.Endharti A. T., Wahyuningtyas T. E., Hardini K. H. Dendrophthoe pentandra leaves extract promotes apoptotic effects of doxorubicin in human breast cancer cell via modulation of intracellular calcium and survivin. Journal of Applied Pharmaceutical Science. 2018;8(08):039–043. [Google Scholar]
- 41.Endharti A. T., Sulastri E., Umanailo R., et al. Mango mistletoe Dendrophthoe pentandra leaf extract acts synergistically with 5-Fluorouracil to induce apoptosis and increase p21 expression in human cervical adenocarcinoma HeLa cells by reducing survivin expression. Journal of Applied Pharmaceutical Science. 2018;8(07):010–015. [Google Scholar]
- 42.Liu C. M., Chen X. T., Pan Y. Y., Liang H., Song S. L., Ji A. G. Antitumor studies of earthworm fibrinolytic enzyme component a from eisenia foetida on breast cancer cell line MCF-7. Indian Journal of Pharmaceutical Sciences. 2017;79(3):361–368. doi: 10.4172/pharmaceutical-sciences.1000238. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
These data used to support the findings of this study are available from the corresponding author upon request.