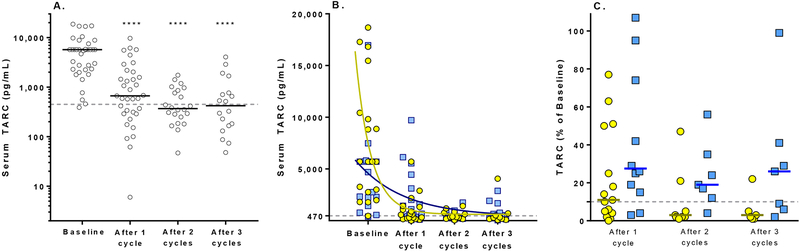
Figure 3. Change in serum TARC after treatment with Brentuximab vedotin and gemcitabine.
A. Serum TARC decreases significantly from baseline, within one cycle of therapy. Each circle represents an individual patient. Solid bars indicate the median value. The dotted green line indicates the upper limit of serum TARC among healthy controls, 470 pg/mL. ****, P<0·0001, compared to baseline. B. Serum TARC after treatment with Brentuximab vedotin and gemcitabine among individual patients who experienced a complete response (yellow circles) vs. remaining patients (progressive disease, partial response, stable disease; blue squares). Solid lines indicate the nonlinear fit of serum TARC data for each group. The dotted gray line indicates the upper limit of serum TARC among healthy controls, 470 pg/mL. C. Change in serum TARC after treatment with Brentuximab vedotin and gemcitabine, shown as % of baseline (100 × serum TARC at the indicated timepoint divided by baseline TARC) among patients with a complete response (open white circles) vs. remaining patients (progressive disease, partial response, stable disease; shaded squares). Lower values indicate a greater reduction from baseline. Values above 100 indicate an increase in serum TARC from baseline. Bold horizontal bars indicate median values. The dotted horizontal line indicates 10% of baseline, i.e. a 90% reduction with therapy.