Figure 3.
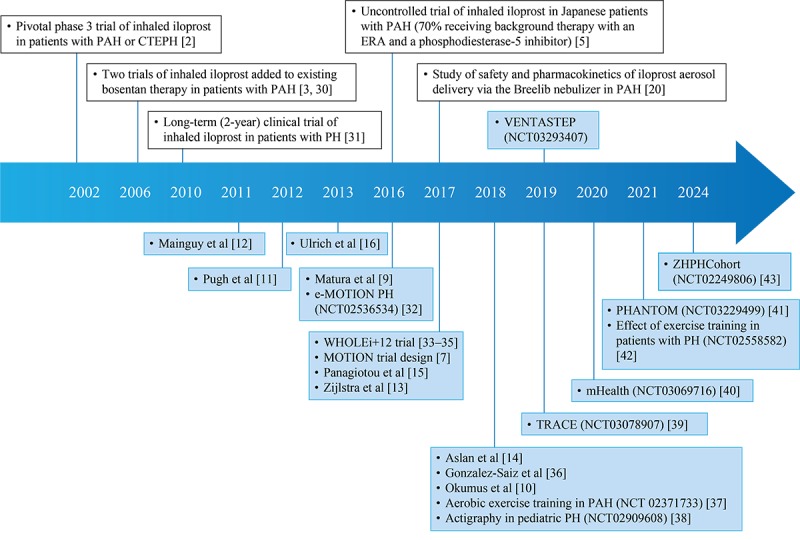
Timeline of key studies of inhaled iloprost and studies of digitally monitored daily physical activity in pulmonary arterial hypertension. Studies of inhaled iloprost (with traditional or digital endpoints) are shown above the timeline, and other studies in pulmonary arterial hypertension with digital monitoring of daily physical activity are shown below the timeline. Blue shading indicates studies with digital monitoring of daily physical activity. Published studies are positioned on the timeline by year of publication; unpublished studies are shown with their ClinicalTrials.gov ID numbers and positioned on the timeline by year of (anticipated) study completion. CTEPH: chronic thromboembolic pulmonary hypertension; e-MOTION PH: electronic activity level monitoring pilot in pulmonary hypertension; ERA: endothelin receptor antagonist; LONGACT: correlation of long-term wrist actigraphy recorded physical performance and 6-min walk distance in patients with pulmonary arterial hypertension; mHealth: mobile health intervention in pulmonary arterial hypertension; MOTION: measuring outcomes in patients with pulmonary arterial hypertension not on active treatment; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; PHANTOM: pulmonary hypertension and anastrozole trial; TRACE: effect of selexipag on daily life physical activity of patients with pulmonary arterial hypertension; VENTASTEP: evaluation of inhaled iloprost effects using the Breelib nebulizer, on clinical outcomes and physical activity of patients with advanced pulmonary arterial hypertension; WHOLEi+12: whole muscle exercise training in pulmonary hypertension; ZHPHCohort: Zürich Pulmonary Hypertension Outcome Assessment Cohort.
