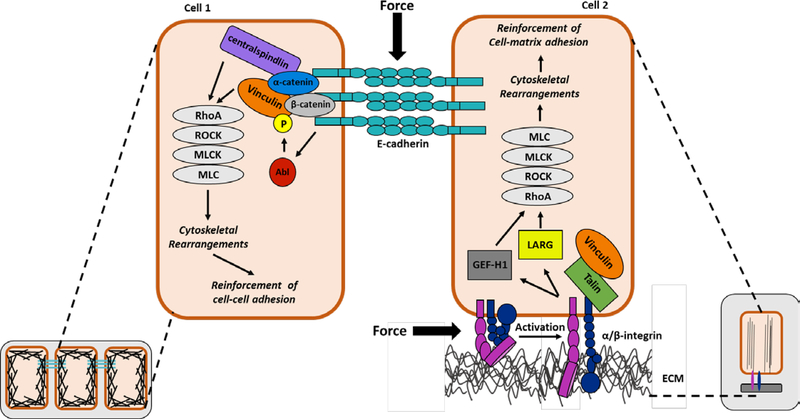
Figure 1: Integrin- and cadherin-mediated mechanotransduction pathways.
At sites of cell-cell contact, the extracellular domains of E-cadherin bind to E-cadherins on neighboring cells to provide strong cell–cell adhesion, while the cytoplasmic domain recruits catenins, which in turn associate with additional cytoskeletal and regulatory proteins, such as vinculin and centralspindlin. In response to force, cadherin induces the activation of Abelson tyrosine kinase (Abl) which leads to the phosphorylation of vinculin at Y822. This signaling event is necessary for the activation of the Rho GTPase pathway, ultimately leading to cadherin-mediated cell stiffening. At cell-matrix adhesions activated integrins interact with the extracellular matrix on the outside of the cell, which triggers activation of intracellular signaling and recruitment of actin binding proteins, such as talin and vinculin. In response to force, the integrins recruit and activate two distinct signaling pathways that trigger recruitment of two RhoA guanine nucleotide exchange factors, LARG and GEF-H1. These integrin-mediated signaling events are critical for the force-induced activation of RhoA and the reinforcement of integrin-actin linkages at the cell-matrix adhesions. Abl=Abelson tyrosine kinase; ROCK=Rho associated protein kinase; MLCK=myosin light chain kinase; MLC=myosin light chain; GEF-H1=guanine nucleotide exchange factor H1; LARG= leukemia-associated Rho-GEF; ECM=extracellular matrix