Figure 3.
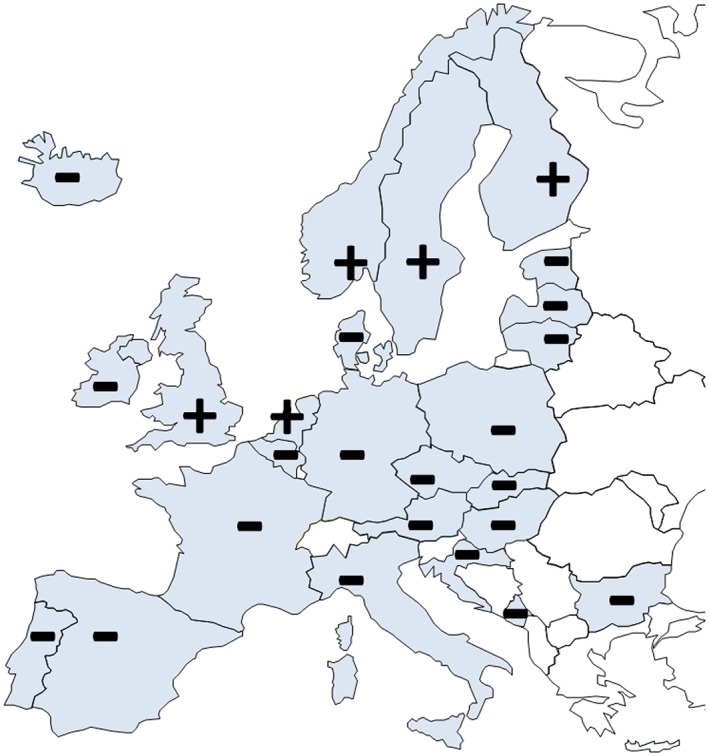
Market authorization for both components H and ISDN. Plus (+) and minus (−) signs depict national market authorization for both components of H–ISDN, H (ATC C02DB02), and ISDN (ATC C01DA08). There is no centralized EU authorization for either drug, and a national market authorization for both components in only 5 of the 25 European nations surveyed (the UK, the Netherlands, and three Nordic countries). Spain only has sublingual isosorbide dinitrate available (5 mg).
