Figure 6. Phenotypic and Spatial Characterization of Human Spinal Cord Neural Stem Cell Grafts into Primates.
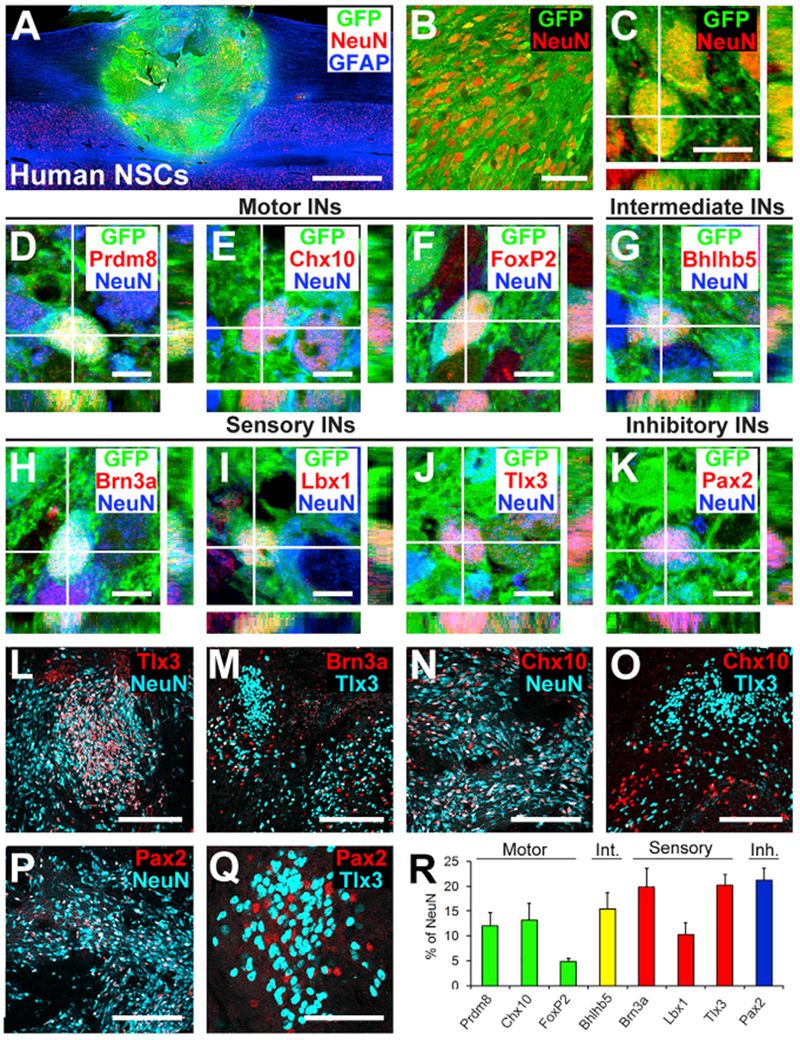
(A) Sagittal overview of human spinal cord-derived neural stem cell (NSC) graft placed in C7 right lateral hemisection lesion cavity in an adult rhesus monkey, 3 month time point. Horizontal section, rostral is left. Graft expresses GFP; section is also labeled for NeuN and GFAP. Graft survives and fills the lesion cavity. Left, rostral; right, caudal. Scale bar, 2 mm.
(B and C) Confocal images of GFP and the neuronal marker NeuN, demonstrating human graft (GFP) differentiation into NeuN-expressing neurons, 3 months after grafting. Scale bars, 10 μm (C) and 50 μm (D).
(D–K) Confocal images of sections labeled for GFP, NeuN, and various interneuronal markers. GFP/NeuN-expressing neurons express the pre-motor interneuronal markers (D) Prdm8 for V0 interneurons, (E) Chx10 for V2a excitatory pre-motor interneurons, (F) FoxP2 for VI inhibitory interneurons, (G) Bhlhb5 for intermediate interneurons (dl6 and V1–2), (H) Brn3a for sensory interneurons (dl1–3), (I) Lbx1 for sensory interneurons (dl4–6), (J) Tlx3 for sensory interneurons (dl3, dlLB, and dl5), and (K) Pax2 for inhibitory interneurons. Scale bars, 10 μm. (L and M) Tlx3- (L) and Brn3a- (M) expressing sensory interneurons are present in clusters in human NSC grafts, similar to observations in rodent models. Scale bars, 200 μm.
(N and O) Chx10-expressing V2a interneurons are present within human NSC grafts (N) and are distributed apart from regions of Tlx3-expressing sensory interneuronal clusters (O). Scale bars, 200 μm.
(P and Q) Pax2-expressing inhibitory interneurons are distributed uniformly in grafts (P) and are also present within sensory interneuronal clusters (Q). Scale bars, 200 μm (P) and 50 μm (Q).
(R) Quantification of neuronal subtypes within human NSC grafts in monkeys (n = 3). Human NSC grafts contain higher proportions of pre-motor interneuronal markers than rat neural stem cell grafts (compare to Figure 1L). Int., intermediate; Inh., inhibitory. Means ± SEM.
