This post hoc analysis of the COMMANDER HF randomized clinical trial examines whether low-dose rivaroxaban was associated with a reduction in thromboembolic events.
Key Points
Question
Did the addition of low-dose rivaroxaban to background antiplatelet therapy reduce risk for thromboembolic events in patients with chronic heart failure and reduced ejection fraction, coronary artery disease, and sinus rhythm?
Findings
In this post hoc analysis of a randomized clinical trial of 5022 patients, although thromboembolic events were not the major driver of the primary efficacy outcome, they occurred frequently. Rivaroxaban was associated with reduced risk of thromboembolic events compared with placebo from 15.5% to 13.1% with inclusion of sudden/unwitnessed deaths as part of the outcome and from 7.6% to 6.1% when sudden/unwitnessed deaths where not considered as part of the thromboembolic composite outcome.
Meaning
Thromboembolic events are common in patients with heart failure, coronary artery disease, and sinus rhythm, and rivaroxaban may reduce risk of their occurrence.
Abstract
Importance
Whether anticoagulation benefits patients with heart failure (HF) in sinus rhythm is uncertain. The COMMANDER HF randomized clinical trial evaluated the effects of adding low-dose rivaroxaban to antiplatelet therapy in patients with recent worsening of chronic HF with reduced ejection fraction, coronary artery disease (CAD), and sinus rhythm. Although the primary end point of all-cause mortality, myocardial infarction, or stroke did not differ between rivaroxaban and placebo, there were numerical advantages favoring rivaroxaban for myocardial infarction and stroke.
Objective
To examine whether low-dose rivaroxaban was associated with reduced thromboembolic events in patients enrolled in the COMMANDER HF trial.
Design, Setting, and Participants
Post hoc analysis of the COMMANDER HF multicenter, randomized, double-blind, placebo-controlled trial in patients with CAD and worsening HF. The trial randomized 5022 patients postdischarge from a hospital or outpatient clinic after treatment for worsening HF between September 2013 and October 2017. Patients were required to be receiving standard care for HF and CAD and were excluded for a medical condition requiring anticoagulation or a bleeding history. Patients were randomized in a 1:1 ratio. Analysis was conducted from June 2018 and January 2019.
Intervention
Patients were randomly assigned to receive 2.5 mg of rivaroxaban given orally twice daily or placebo in addition to their standard therapy.
Main Outcomes and Measures
For this post hoc analysis, a thromboembolic composite was defined as either (1) myocardial infarction, ischemic stroke, sudden/unwitnessed death, symptomatic pulmonary embolism, or symptomatic deep venous thrombosis or (2) all of the previous components except sudden/unwitnessed deaths because not all of these are caused by thromboembolic events.
Results
Of 5022 patients, 3872 (77.1%) were men, and the overall mean (SD) age was 66.4 (10.2) years. Over a median (interquartile range) follow-up of 19.6 (11.7-30.8) months, fewer patients assigned to rivaroxaban compared with placebo had a thromboembolic event including sudden/unwitnessed deaths: 328 (13.1%) vs 390 (15.5%) (hazard ratio, 0.83; 95% CI, 0.72-0.96; P = .01). When sudden/unwitnessed deaths were excluded, the results analyzing thromboembolic events were similar: 153 (6.1%) vs 190 patients (7.6%) with an event (hazard ratio, 0.80; 95% CI, 0.64-0.98; P = .04).
Conclusions and Relevance
In this study, thromboembolic events occurred frequently in patients with HF, CAD, and sinus rhythm. Rivaroxaban may reduce the risk of thromboembolic events in this population, but these events are not the major cause of morbidity and mortality in patients with recent worsening of HF for which rivaroxaban had no effect. While consistent with other studies, these results require confirmation in prospective randomized clinical trials.
Trial Registration
ClinicalTrials.gov identifier: NCT01877915
Introduction
Low-dose rivaroxaban improved outcomes including survival in patients with acute coronary syndrome (ACS) in the Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction 51 (ATLAS ACS 2–TIMI 51) study1 and in stable patients with chronic atherosclerotic cardiovascular (CV) disease in the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial.2 A post hoc analysis of results from the ATLAS ACS 2–TIMI 51 study1 and a prespecified analysis of the COMPASS trial2 showed that the beneficial effects of rivaroxaban on reduction of major adverse CV events (MACE), defined as a composite of CV death, myocardial infarction (MI), or stroke, were at least as great in patients with a history of heart failure (HF) as in those without HF.3,4 However, in the COMMANDER HF trial (A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants with Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure),5 which enrolled patients after a recent worsening of chronic HF, coronary artery disease (CAD), and normal sinus rhythm, low-dose rivaroxaban did not significantly reduce the risk of the primary composite outcome of all-cause mortality, MI or stroke, all-cause mortality alone, or the main secondary efficacy outcome of CV death and HF hospitalization.5
The reasons for the different findings in the patients with HF in ATLAS ACS 2–TIMI 51,1 COMPASS,2 and COMMANDER HF5 studies are not clear. Although patients enrolled in all 3 trials were required to have CAD or atherosclerotic vascular disease and to be in sinus rhythm, only the COMMANDER HF trial5 required patients to have had a recent episode of worsening HF for entry into the study. Not surprisingly, given the known increase in the risk of death after an episode of worsening HF,6,7,8,9 mortality rates varied considerably between the studies, with all-cause and CV mortality both considerably higher in the COMMANDER HF trial5 than in either the overall study populations1,2 or in the subgroups of patients with HF in ATLAS ACS 2–TIMI 513 or COMPASS.4 This led us to hypothesize that the contrasting results for the HF subgroups between the studies might be due to the fact that many of the deaths in the COMMANDER HF trial5 were not amenable to antithrombotic therapy as they were associated with pump failure. Regardless, there is evidence that patients with HF and CAD are at increased risk for atherothrombotic events,10,11,12,13,14,15,16 and it is possible that an effect of rivaroxaban on thromboembolic events in the COMMANDER HF trial5 might have been masked by pump failure deaths that drove the primary outcome. Thus, for this post hoc analysis, we defined an outcome that was more specific to thromboembolic events.
Methods
Study Population
The design and primary results for this study have been previously published.5,17 Briefly, the COMMANDER HF trial was a multicenter, randomized, double-blind, placebo-controlled trial that enrolled patients who had a history of chronic HF and had been treated for an episode of worsening HF within the previous 21 days, a left ventricular ejection fraction of 40% or less, a history of CAD, and were in normal sinus rhythm.
Patients were assigned in a 1:1 proportion to receive 2.5 mg twice daily (low-dose) rivaroxaban or matching placebo. All patients received standard care for HF and CAD as prescribed by their treating physician. After randomization, patients were followed up at weeks 4 and 12 and then every 12 weeks thereafter for assessment of safety and to ascertain the occurrence of outcome events. All participants provided written informed consent. The study protocol was approved by the ethics committee/institutional review board at each site. The trial was conducted and reported in accordance with the protocol (Supplement 1) and the statistical analysis plan (Supplement 2). This analysis was completed with existing deidentified data from the COMMANDER HF trial, which did not require separate approval.
Efficacy Outcome Events
Sites evaluated patients for the occurrence of an efficacy outcome event at each visit. Events reported by investigators during the trial included death, MI, stroke, symptomatic pulmonary embolism (PE), and symptomatic deep vein thrombosis. Strokes were further subdivided according to whether they were hemorrhagic or of ischemic etiology based on predefined criteria. Investigators determined the primary cause of death and classified each death as CV, non-CV, or unknown.
Although an independent clinical events adjudication committee was not used in the COMMANDER HF trial, the outcome event definitions and criteria required for documentation of an event were provided in rigorous detail in the protocol and in the eAppendix in Supplement 3. Each type of efficacy outcome had a specific case report form on which investigators documented the information relevant to the event, included the criteria that had been met, and had an assessment of the available source documentation. The protocol contained predefined criteria for each outcome event that had to be documented by the investigator with medical records, which could include (as examples) imaging and laboratory reports, consultation reports, or discharge summaries. Moreover, documentation was carefully reviewed by the local trial monitor and sent to the sponsor’s blinded clinical group for confirmatory review. If these reports met the protocol-required criteria, the event was considered verified.
Thromboembolic events included in this post hoc analysis were defined in 2 ways (Table 1). Both definitions included MI, ischemic stroke, symptomatic PE, and symptomatic deep vein thrombosis as components of the thromboembolic composite. One of the definitions also included sudden/unwitnessed deaths as part of the composite. Cardiovascular deaths (eg, HF, cardiogenic shock) were excluded if they were not reported as sudden/unwitnessed as our goal was to focus on events associated with a thromboembolic mechanism.
Table 1. Thromboembolic Event Definitions.
| Thromboembolic Event Typea | Definitionb |
|---|---|
| Myocardial infarctionc |
|
| Ischemic strokec | New, sudden, focal neurological deficit resulting from a presumed cerebrovascular cause that is not reversible within 24 h and not due to readily identifiable cause, such as trauma, a tumor, or seizure; no focal collections of intracranial blood |
| Sudden/unwitnessed death |
|
| Symptomatic PE |
|
| Symptomatic DVT | Symptoms of DVT in the extremity in question and positive compression ultrasonography or venogram |
Abbreviations: CABG, coronary artery bypass grafting; CT, computed tomography; CV, cardiovascular; DVT, deep vein thrombosis; LBBB, left bundle branch block; MI, myocardial infarction; PCI, percutaneous coronary intervention; PE, pulmonary embolism.
Thromboembolic outcomes were defined in 2 ways: (1) MI, ischemic stroke, sudden/unwitnessed death, symptomatic pulmonary embolism, or symptomatic deep venous thrombosis and (2) all of the previous events except sudden/unwitnessed deaths.
Complete efficacy event definitions are in the trial protocol in Supplement 1 and described elsewhere.17
Fatal MI and stroke events were determined by the investigator.
We defined 2 additional outcomes: (1) MACE and (2) nonthromboembolic events (ie, deaths not attributable to sudden/unwitnessed cause and hemorrhagic stroke). The former is defined for comparison with ATLAS ACS 2–TIMI 51,1 and the latter to assess the association of rivaroxaban with major events not considered to be thromboembolic.
Statistical Analysis
For this post hoc analysis, time-to-event methods were used for comparing the rivaroxaban and placebo groups for the composite thromboembolic outcome and the components of this outcome, ie, a Cox model stratified by 5 geographic regions. Hazard ratios (HRs), 95% confidence intervals, and 2-sided P values with a significance threshold of P < .05 are cited. Similar methods were used to compare the 2 treatment groups for MACE and for the composite nonthromboembolic outcome. To assess whether the HRs for the components of the composite outcomes defined varied, marginal associations with each component were estimated using all the first component event(s) experienced by each patient. We compared the fit of a model that allowed the associations to vary with that of a model that assumed a common association. A common treatment effect was also estimated by the Wei-Lin-Weissfeld model,18 an extension of the Cox regression model to account for the correlation of multiple events within a patient. Because of the post hoc nature of these analyses, P values should be cautiously interpreted and used primarily to generate hypotheses to be tested in future studies.
Results
Detailed baseline characteristics of the patients in the COMMANDER HF trial have been reported elsewhere.5 Table 2 gives demographic and selected other baseline characteristics that reflect severity of HF, potential risk factors for thromboembolic events, and use of agents that might affect these events. Overall, 3872 of 5022 patients (77.1%) were men, 4128 (82.2%) were white, and the mean (SD) age was 66.4 (10.2) years. Overall, 2654 patients (52.8%) were categorized as being in New York Heart Association class III or IV; median (interquartile) baseline ejection fraction was 34% (28%-38%). A history of MI or stroke was present in 3803 (75.7%) and 453 (9.0%) patients, respectively. Antiplatelet agents were prescribed for virtually all patients, with 4675 (93.1%) receiving aspirin, 2015 (40.1%) receiving thienopyridines, and 1746 (34.8%) receiving dual antiplatelet therapy.
Table 2. Selected Baseline Variables in Patients Enrolled in the COMMANDER HF Triala.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Rivaroxaban (n = 2507) | Placebo (n = 2515) | Total (N = 5022) | |
| Age, mean (SD), y | 66.51 (10.07) | 66.28 (10.27) | 66.40 (10.17) |
| Women | 551 (22.0) | 599 (23.8) | 1150 (22.9) |
| Whiteb | 2063 (82.3) | 2065 (82.1) | 4128 (82.2) |
| Medical history | |||
| Myocardial infarction | 1911 (76.2) | 1892 (75.2) | 3803 (75.7) |
| Stroke | 208 (8.3) | 245 (9.7) | 453 (9.0) |
| Hypertension | 1897 (75.7) | 1886 (75.0) | 3783 (75.3) |
| Diabetes | 1024 (40.8) | 1028 (40.9) | 2052 (40.9) |
| Clinical features of heart failure, median (IQR) | |||
| BNP, pg/mLc | 702.0 (403.4 -1237.0) | 695.6 (380.0-1266.3) | 700.0 (390.0-1250.0) |
| NT-proBNP, pg/mLc | 2480.0 (1537.0-6394.0) | 2900.0 (1520.0-6270.5) | 2873.5 (1531.0-6322.0) |
| D-dimer, ug/L | 360.0 (215.0-680.0) | 360.0 (215.0-650.0) | 360 (215.0-665.0) |
| Ejection fraction | 35 (28-38) | 34 (27-38) | 34 (28-38) |
| New York Heart Association classification | |||
| I | 80 (3.2) | 69 (2.7) | 149 (3.0) |
| II | 1122 (44.8) | 1096 (43.6) | 2218 (44.2) |
| III | 1208 (48.2) | 1254 (49.9) | 2462 (49.0) |
| IV | 96 (3.8) | 96 (3.8) | 192 (3.8) |
| Therapies at baseline | |||
| Aspirin | 2329 (92.9) | 2346 (93.3) | 4675 (93.1) |
| Thienopyridine | 1043 (41.6) | 972 (38.6) | 2015 (40.1) |
| Dual antiplatelet therapy | 907 (36.2) | 839 (33.4) | 1746 (34.8) |
| Cardiac devicesd | 345 (13.8) | 316 (12.6) | 661 (13.2) |
Abbreviations: BNP, brain-type natriuretic peptide; IQR, interquartile range; NT-proBNP, amino-terminal pro-B-type natriuretic peptide.
SI conversion factors: To convert BNP from pg/mL to ng/L, multiply by 1.0; to convert D-dimer from ug/L to nmol/L, multiply by 3.04.
The complete list of baseline characteristics has been previously published.5
Race/ethnicity was self-reported by the patient.
After the enrollment of 1155 patients (23.0%), a protocol amendment required patients to also have a plasma concentration of BNP that was at least 200 pg/mL or NT-proBNP that was at least 800 pg/mL. Brain-type natriuretic peptide values were provided for 965 patients, and NT-proBNP values were provided for 2862 patients.
Cardiac devices include cardiac resynchronization therapy, implantable cardioverter defibrillator, and pacemaker.
Table 3 summarizes the treatment differences for the primary end point of COMMANDER HF (for reference) and the post hoc analyses of thromboembolic, nonthromboembolic, and MACE. As previously reported, 26% of patients experienced a primary end point in COMMANDER HF. Most primary events were deaths (84% CV death [n = 929], 11% non-CV death [n = 121], and 5% unknown death [n = 52]) that were not preceded by an MI or stroke. The HR (rivaroxaban vs placebo) was 0.94 (95% CI, 0.84-1.05; P = .27). However, differences in the HR between the components were apparent, ranging from 0.98 (95% CI, 0.87-1.10) for mortality to 0.66 (95% CI, 0.47-0.95) for stroke. The P value for the test of homogeneity of the 3 components of the composite primary end point was .06. The pooled HR based on the marginal model was the same as the model based on the first event (HR, 0.94).
Table 3. Time to First Occurrence of an Outcome Eventa.
| Outcomesb | Rivaroxaban (n = 2507) | Placebo (n = 2515) | Rivaroxaban vs Placeboc | |||
|---|---|---|---|---|---|---|
| No. (%) | Event Rate/100 Patient-Year | No. (%) | Event Rate/100 Patient-Year | Hazard Ratio (95% CI) | P Valued | |
| Primary end point (composite of ACM/MI/stroke) | 626 (25.0) | 13.4 | 658 (26.2) | 14.3 | 0.94 (0.84-1.05) | .27 |
| ACM | 546 (21.8) | 11.4 | 556 (22.1) | 11.6 | 0.98 (0.87-1.10) | NA |
| MI | 98 (3.9) | 2.1 | 118 (4.7) | 2.5 | 0.83 (0.63-1.08) | |
| Stroke | 51 (2.0) | 1.1 | 76 (3.0) | 1.6 | 0.66 (0.47-0.95) | |
| Composite of thromboembolic events | 328 (13.1) | 7.0 | 390 (15.5) | 8.5 | 0.83 (0.72-0.96) | .01 |
| MI | 98 (3.9) | 2.1 | 118 (4.7) | 2.5 | 0.83 (0.63-1.08) | NA |
| Ischemic stroke | 41 (1.6) | 0.9 | 63 (2.5) | 1.3 | 0.64 (0.43-0.95) | |
| Sudden/unwitnessed death | 190 (7.6) | 4.0 | 215 (8.5) | 4.5 | 0.88 (0.73-1.07) | |
| Symptomatic PE | 11 (0.4) | 0.2 | 9 (0.4) | 0.2 | 1.24 (0.51-2.99) | |
| Symptomatic hazard ratio | 5 (0.2) | 0.1 | 7 (0.3) | 0.1 | 0.71 (0.23-2.24) | |
| Composite of thromboembolic events excluding sudden/unwitnessed deaths | 153 (6.1) | 3.3 | 190 (7.6) | 4.1 | 0.80 (0.64-0.98) | .04 |
| Composite of nonthromboembolic events | 363 (14.5) | 7.6 | 346 (13.8) | 7.2 | 1.05 (0.91-1.22) | .51 |
| Death other than CV deathe | 93 (3.7) | 1.9 | 80 (3.2) | 1.7 | 1.16 (0.86-1.57) | NA |
| CV death other than sudden/unwitnessed death | 263 (10.5) | 5.5 | 261 (10.4) | 5.5 | 1.01 (0.85-1.19) | |
| Nonischemic stroke | 11 (0.4) | 0.2 | 13 (0.5) | 0.3 | 0.85 (0.38-1.90) | |
| Composite of CV death, MI, or stroke | 537 (21.4) | 11.5 | 584 (23.2) | 12.7 | 0.91 (0.81-1.02) | .11 |
| CV death | 453 (18.1) | 9.5 | 476 (18.9) | 10.0 | 0.95 (0.84-1.08) | NA |
| MI | 98 (3.9) | 2.1 | 118 (4.7) | 2.5 | 0.83 (0.63-1.08) | |
| Stroke | 51 (2.0) | 1.1 | 76 (3.0) | 1.6 | 0.66 (0.47-0.95) | |
| Composite of MI or ischemic stroke | 137 (5.5) | 2.9 | 176 (7.0) | 3.8 | 0.77 (0.62-0.96) | .02 |
| MI | 98 (3.9) | 2.1 | 118 (4.7) | 2.5 | 0.83 (0.63-1.08) | NA |
| Ischemic stroke | 41 (1.6) | 0.9 | 63 (2.5) | 1.3 | 0.64 (0.43-0.95) | |
Abbreviations: ACM, all-cause mortality; CV, cardiovascular; MI, myocardial infarction; NA, not applicable; PE, pulmonary embolism.
For the composite outcome, only the first event in a given patient was included; for the individual components of that outcome, all first events of that component outcome were included.
Data are from the intention-to-treat population during the observation period from randomization through the global treatment end date (March 5, 2018).
Hazard ratios and 95% CIs are from a Cox proportional hazards model stratified according to region, with trial group assignment as the only effect. P value (2-sided) is from the log-rank test stratified according to region. The 95% CIs have not been adjusted for multiplicity, and inferences drawn from these intervals may not be reproducible.
P values are given for the composite outcomes but not the components that define each composite.
Including non-CV death and unknown death.
When sudden/unwitnessed deaths were considered as part of the composite, 718 of 5022 patients (14%) in COMMANDER HF experienced a thromboembolic event (Table 3). These events occurred in 328 patients (13.1%) assigned to rivaroxaban and 390 patients (15.5%) assigned to placebo (HR, 0.83; 95% CI, 0.72-0.96; P = .01). With the exception of symptomatic PE, for which the number of patients with an event was small, components favored rivaroxaban over placebo, with HRs ranging from 0.64 for stroke to 0.88 for sudden/unwitnessed death (P = .60 for homogeneity of HRs for the 5 components of this composite outcome). When sudden/unwitnessed deaths were excluded from the thromboembolic composite outcome, 153 patients (6.1%) assigned rivaroxaban and 190 patients (7.6%) assigned to placebo experienced a thromboembolic event (HR, 0.80; 95% CI, 0.64-0.98; P = .04); (P = .56 for homogeneity of 4 components of this composite).
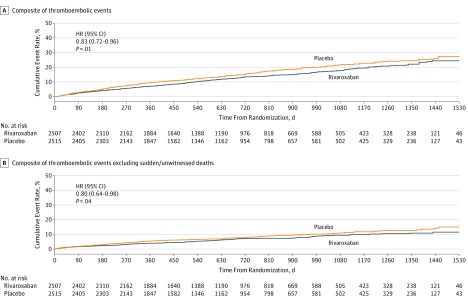
The Kaplan-Meier curves for the composite thromboembolic outcome with and without sudden/unwitnessed deaths are shown in Figure A and B, respectively. Curves for MI and ischemic stroke are shown in eFigures 1 and 2 in Supplement 3.
Figure. Time to First Occurrence of Thromboembolic Events.
A, The composite of thromboembolic events includes myocardial infarction, ischemic stroke, sudden/unwitnessed death, symptomatic pulmonary embolism, or symptomatic deep vein thrombosis. The cumulative percentage of patients with a thromboembolic event were 7%, 14%, and 18% after approximately 12, 24, and 36 months, respectively, among those assigned to rivaroxaban and 10%, 16%, and 22%, respectively, among those assigned to placebo. B, The composite of thromboembolic events includes myocardial infarction, ischemic stroke, symptomatic pulmonary embolism, or symptomatic deep vein thrombosis. The cumulative percentage of patients with a thromboembolic event excluding sudden/unwitnessed deaths was 4%, 7%, and 9% after 12, 24, and 36 months, respectively, for those patients assigned to rivaroxaban, and 5%, 8%, and 11%, respectively, for those assigned to placebo. HR indicates hazard ratio.
Nonthromboembolic components of the primary end point of COMMANDER HF occurred with similar frequency as the thromboembolic components (709 patients [14.1%]: 363 patients (14.5%) assigned to rivaroxaban and 346 patients (13.8%) assigned to placebo; Table 3). Most of these events were death, 75% (n = 524) attributed to CV death and 25% (n = 173) attributed to non-CV death, including death from unknown causes. Cause-specific mortality by treatment is shown in the eTable in Supplement 3. The HR for nonthromboembolic events was 1.05 (95% CI, 0.91-1.22; P = .51) (P = .64 for homogeneity of 3 components of this composite).
The primary efficacy end point used in the ATLAS ACS 2–TIMI 51 trial,1 MACE, occurred in 1121 patients (22%), which included 537 patients (21.4%) assigned to rivaroxaban and 584 patients (23.2%) assigned to placebo (Table 3). The HR for MACE was 0.91 (95% CI, 0.81-1.02; P = .11). The P value for homogeneity of the MACE components was .11. Like the primary end point for COMMANDER HF, the pooled HR based on the marginal model for all reported events was identical (HR, 0.91).
Discussion
Although there was no difference in efficacy between rivaroxaban and placebo in COMMANDER HF for the primary composite end point, HRs for the components varied, with numerical advantages observed for rivaroxaban compared with placebo for MI and stroke, both of which involve a thromboembolic mechanism. Consequently, this post hoc analysis was performed to assess the risk of thromboembolic events in COMMANDER HF and to determine whether the risk of these events might be reduced by low-dose rivaroxaban therapy. We found that rivaroxaban reduced the rate of thromboembolic events by 17% compared with placebo. While these findings are encouraging and consistent with those from earlier studies,1,2 they are by no means conclusive and will require confirmation in future randomized clinical trials.
The COMMANDER HF trial was designed to determine if low-dose rivaroxaban could improve outcomes in patients who had experienced recent worsening of chronic HF, CAD, and normal sinus rhythm. Previous studies that assessed the effects of anticoagulation on outcomes in patients with HF who were in sinus rhythm provided inconclusive results.19,20,21,22,23 A post hoc analysis of patients included in the Studies of Left Ventricular Dysfunction19 reported that warfarin was associated with improved survival, but prospective studies comparing warfarin with antiplatelet agents failed to confirm this benefit.20,21,22,23 In the Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) Trial,23 which was the largest and most informative of these trials, although a net benefit of warfarin therapy compared with aspirin was not observed, there was a reduction in ischemic stroke with warfarin but this was offset by an increased risk of major hemorrhage.23 More recently, at the time that COMMANDER HF was designed, data from the ATLAS ACS 2–TIMI 51 trial1 indicated that low-dose rivaroxaban reduced the risk of MACE from a subgroup of patients with HF included in that trial.3 This finding, along with evidence that thrombin-mediated events are involved in the progression of HF,10,11,12,13,14,15,16,20,21,22,23,24,25,26,27,28 was the rationale for the COMMANDER HF trial. The patients enrolled and the therapeutic intervention used in COMMANDER HF differed considerably from previous trials, such as the WARCEF study,23 in that COMMANDER HF required patients to have a history of CAD for entry and to have experienced recent worsening of chronic HF. While the WARCEF trial23 compared the effects of warfarin with those of aspirin, in COMMANDER HF, antiplatelet therapy (including aspirin in 93.1%) was universal. In addition, WARCEF23 used full-dose anticoagulation, and COMMANDER HF used low-dose rivaroxaban. The failure of low-dose rivaroxaban to alter either the primary efficacy end point, all-cause mortality alone, which occurred in 22% of patients, or the secondary composite end point of CV mortality or HF rehospitalization outcomes, which occurred in 37% of patients in COMMANDER HF, suggests that thromboembolic events are not the major driver of outcomes in patients with worsening HF and reduced ejection fraction.
As part of these post hoc analyses, we evaluated the association of rivaroxaban with MACE in COMMANDER HF for comparison with patients with HF who were included in the ATLAS ACS 2–TIMI 51 trial.3 When MACE events were compared between patients in COMMANDER HF and patients with HF in ATLAS ACS 2–TIMI 513 (Table 4), many more patients experienced CV death as part of the MACE composite in COMMANDER HF than in ATLAS ACS 2–TIMI 51.3 While risk reductions with rivaroxaban for MI and stroke in COMMANDER HF, patients were similar to those seen in patients with HF in ATLAS ACS 2–TIMI 51,3 the composite outcome in COMMANDER HF, which was largely determined by CV death, was not. In COMPASS,2 the rate of MACE was significantly lower with the combination of rivaroxaban plus aspirin vs aspirin alone, and a recent analysis suggested that this was also evident for the subgroup of patients with HF in the COMPASS population.4
Table 4. Comparison of Outcomes for Patients With Heart Failure in COMMANDER HF vs ATLAS ACS 2–TIMI 51 Studiesa.
| Variable | COMMANDER HF | ATLAS ACS 2–TIMI 51 (HF subgroup)3 | ||||
|---|---|---|---|---|---|---|
| No. (%) | HR (95% CI) | No. (%) | HR (95% CI) | |||
| 2.5 mg of Rivaroxaban Twice/d (n = 2507) | Placebo (n = 2515) | 2.5 mg of Rivaroxaban Twice/d (n = 562) | Placebo (n = 558) | |||
| Composite of CV death, MI, or stroke | 537 (21.4) | 584 (23.2) | 0.91 (0.81-1.02) | 59 (10.5) | 96 (17.2) | 0.59 (0.42-0.81) |
| CV death | 453 (18.1) | 476 (18.9) | 0.95 (0.84-1.08) | 23 (4.1) | 50 (9.0) | 0.45 (0.27-0.74) |
| MI | 98 (3.9) | 118 (4.7) | 0.83 (0.63-1.08) | 36 (6.4) | 50 (9.0) | 0.68 (0.44-1.04) |
| Stroke | 51 (2.0) | 76 (3.0) | 0.66 (0.47-0.95) | 7 (1.7) | 10 (1.8) | 0.68 (0.26-1.80) |
Abbreviations: CV, cardiovascular; HF, heart failure; HR, hazard ratio; MI, myocardial infarction.
An individual could have more than 1 component event.
Taken together with our exploration of a thromboembolic outcome, it is possible that the difference observed in MACE between COMMANDER HF and these 2 studies is because of the inclusion of patients with recent worsening of chronic HF in COMMANDER HF and that more CV deaths in COMMANDER HF were associated with pump failure causes that were not amenable to antithrombotic therapy. Our results also support the possibility that the preponderance of mortality in the primary end point in COMMANDER HF may have masked a favorable association of rivaroxaban and thromboembolic events, which were ongoing in this population, albeit at a lower level than were HF-related events.
While MI, ischemic stroke, symptomatic PE, and symptomatic deep vein thrombosis are considered thromboembolic events, the inclusion of sudden or unwitnessed death is less straightforward. There is evidence suggesting that many of these events are caused by unrecognized MI, microthrombi in the distal coronary vasculature that precipitate an arrhythmia, or PE.10,11,13,15,16 Uretsky et al11 evaluated the prevalence of acute coronary findings (defined as coronary thrombosis, ruptured plaque, or acute MI) at autopsy in the hearts of patients who had died suddenly in the ATLAS ACS 2–TIMI 51 trial,1 a study that, like COMMANDER HF, was carried out in a population with HF with reduced ejection fraction. They found that acute coronary findings were present in 54% of the patients with underlying CAD who had died suddenly compared with only 5% in patients who died suddenly but were without CAD. Similar results were reported by Farb et al13 who assessed the postmortem anatomy of 90 hearts from patients who had died suddenly and found evidence of coronary thrombosis, plaque disruption, or both in 57% of these patients. There is also an association between epicardial CAD and coronary microemboli, which can result in lethal arrhythmias. In a study of 44 hearts of patients who had died suddenly and had evidence of coronary thrombosis, Schwartz et al15 found that there were plaque ruptures in 25 of the hearts and plaque erosions in the remaining 19. Microemboli and microvascular obstruction were found in 54% of these hearts with a mean of 4.5 microemboli per heart. However, other causes of sudden or unwitnessed death including primary arrhythmias are possible, leaving the role of thrombus formation in cases of sudden cardiac death uncertain in many cases. Recognizing this, we defined our thromboembolic composite in 2 ways. Hazard ratios for the thromboembolic composites including and excluding sudden/unwitnessed death were similar (0.83 and 0.80, respectively).
It is important that when using composite outcomes, the individual components are assessed as they may be influenced differentially by the intervention. That appears to be the case in COMMANDER HF. The hypothesis of COMMANDER HF was that rivaroxaban, through inhibition of thrombin, and its influence on inflammation, ischemia, and endothelial dysfunction would prevent HF progression. We anticipated that it would influence equally death associated with HF progression as well as classical atherothromboembolic events, such as stroke and MI, and most deaths would be CV so that adjudication of cause was not required, hence the choice of the trial primary end point of all-cause death, stroke, or MI. The main results of COMMANDER HF proved this hypothesis wrong. However, the results that we present suggest that although rivaroxaban was ineffective in preventing HF-related death, it may have been associated with reduced risk of the thromboembolic-specific end point explored in this analysis. In assessing the potential therapeutic value of low-dose rivaroxaban in the HF population, it is necessary to also recognize the potential risk of this therapy. In COMMANDER HF, the principal safety outcome of fatal bleeding or bleeding into a critical space with the potential for causing permanent disability was not significantly increased in patients assigned to low-dose rivaroxaban, although as detailed in the primary outcome article, there was increase in secondary bleeding outcomes.5
Limitations
Although each of the outcomes assessed in this post hoc analysis had been defined in the study protocol, there was no prespecified plan to assess thromboembolic events using this composite end point. Thus, our findings should be considered hypothesis generating and require confirmation in prospectively planned studies. However, it is worth noting that the reductions in risk for MI and stroke in COMMANDER HF are consistent with the results from ATLAS ACS 2–TIMI 511 and COMPASS,2 both of which included patients with CAD and in the subgroups of patients with HF who were included in these studies. Another limitation is that the end points in COMMANDER HF were not adjudicated by an independent committee. However, they were clearly defined in the study protocol and in an investigator manual for outcome events and they were carefully reviewed. Finally, entry into COMMANDER HF required patients to have a reduced ejection fraction so that findings presented in this analysis may not pertain to those who have HF with preserved ejection fraction.
Conclusions
While the COMMANDER HF study failed to demonstrate a reduction in risk of the composite primary end point of all-cause mortality, MI, or stroke, this analysis shows that thromboembolic events, while not the major source of morbidity and mortality in COMMANDER HF, represent a substantial proportion of events that occurred during follow-up. The incidence of MI and stroke seen in the COMMANDER HF patients was similar to that seen in patients in ATLAS ACS 2–TIMI 511 and COMPASS,2 indicating an ongoing risk for these events across a broad spectrum of patients with CAD. Our findings support the possibility that low-dose rivaroxaban may reduce the risk of these events in patients with HF, as has been reported in ATLAS ACS 2–TIMI 511 and COMPASS.2 However, confirmation in prospective randomized clinical trials that rivaroxaban can reduce the risk of thromboembolic events in patients with HF is still needed to establish a role for this therapy in the treatment of patients with HF.
Trial protocol.
Statistical analysis plan
eTable. Cause-Specific Mortality by Treatment Group-GTED (Intent-To-Treat Analysis Set)
eFigure 1. Kaplan-Meier Estimates for Myocardial Infarction
eFigure 2. Kaplan-Meier Estimates for Ischemic Stroke
eAppendix. Investigator Manual for Outcome Events RIVAROXHFA3001 The COMMANDER HF Study
References
- 1.Mega JL, Braunwald E, Wiviott SD, et al. ; ATLAS ACS 2–TIMI 51 Investigators . Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366(1):9-19. doi: 10.1056/NEJMoa1112277 [DOI] [PubMed] [Google Scholar]
- 2.Eikelboom JW, Connolly SJ, Bosch J, et al. ; COMPASS Investigators . Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319-1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 3.Korjian S, Braunwald E, Daaboul Y, et al. Usefulness of rivaroxaban for secondary prevention of acute coronary syndrome in patients with history of congestive heart failure (from the ATLAS-ACS-2 TIMI-51 Trial). Am J Cardiol. 2018;122(11):1896-1901. doi: 10.1016/j.amjcard.2018.08.034 [DOI] [PubMed] [Google Scholar]
- 4.Branch KR. Rivaroxaban plus aspirin in patients with and without heart failure and chronic coronary or peripheral artery disease: the COMPASS trial. Paper resented at: Heart Failure 2018 & World Congress on Acute Heart Failure; May 28, 2018; Vienna, Austria. [Google Scholar]
- 5.Zannad F, Anker SD, Byra WM, et al. ; COMMANDER HF Investigators . Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med. 2018;379(14):1332-1342. doi: 10.1056/NEJMoa1808848 [DOI] [PubMed] [Google Scholar]
- 6.Lee DS, Austin PC, Stukel TA, et al. “Dose-dependent” impact of recurrent cardiac events on mortality in patients with heart failure. Am J Med. 2009;122(2):162-169.e1. doi: 10.1016/j.amjmed.2008.08.026 [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306(15):1669-1678. doi: 10.1001/jama.2011.1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonarow GC, Stough WG, Abraham WT, et al. ; OPTIMIZE-HF Investigators and Hospitals . Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50(8):768-777. doi: 10.1016/j.jacc.2007.04.064 [DOI] [PubMed] [Google Scholar]
- 9.Solomon SD, Dobson J, Pocock S, et al. ; Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) Investigators . Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116(13):1482-1487. doi: 10.1161/CIRCULATIONAHA.107.696906 [DOI] [PubMed] [Google Scholar]
- 10.Bettari L, Fiuzat M, Becker R, Felker GM, Metra M, O’Connor CM. Thromboembolism and antithrombotic therapy in patients with heart failure in sinus rhythm: current status and future directions. Circ Heart Fail. 2011;4(3):361-368. doi: 10.1161/CIRCHEARTFAILURE.110.959957 [DOI] [PubMed] [Google Scholar]
- 11.Uretsky BF, Thygesen K, Armstrong PW, et al. Acute coronary findings at autopsy in heart failure patients with sudden death: results from the assessment of treatment with lisinopril and survival (ATLAS) trial. Circulation. 2000;102(6):611-616. doi: 10.1161/01.CIR.102.6.611 [DOI] [PubMed] [Google Scholar]
- 12.Loh E, Sutton MS, Wun CC, et al. Ventricular dysfunction and the risk of stroke after myocardial infarction. N Engl J Med. 1997;336(4):251-257. doi: 10.1056/NEJM199701233360403 [DOI] [PubMed] [Google Scholar]
- 13.Farb A, Tang AL, Burke AP, Sessums L, Liang Y, Virmani R. Sudden coronary death: frequency of active coronary lesions, inactive coronary lesions, and myocardial infarction. Circulation. 1995;92(7):1701-1709. doi: 10.1161/01.CIR.92.7.1701 [DOI] [PubMed] [Google Scholar]
- 14.Acharya T, Aspelund T, Jonasson TF, et al. Association of unrecognized myocardial infarction with long-term outcomes in community-dwelling older adults: the ICELAND MI study. JAMA Cardiol. 2018;3(11):1101-1106. doi: 10.1001/jamacardio.2018.3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz RS, Burke A, Farb A, et al. Microemboli and microvascular obstruction in acute coronary thrombosis and sudden coronary death: relation to epicardial plaque histopathology. J Am Coll Cardiol. 2009;54(23):2167-2173. doi: 10.1016/j.jacc.2009.07.042 [DOI] [PubMed] [Google Scholar]
- 16.Schmemund SA, Schwartz RS, Adamzik M, et al. Coronary atherosclerosis in unheralded sudden coronary death under age 50: histo-pathologic comparison with 'healthy' subjects dying out of hospital. Atherosclerosis. 2001;155:499-508. [DOI] [PubMed] [Google Scholar]
- 17.Zannad F, Greenberg B, Cleland JGF, et al. Rationale and design of a randomized, double-blind, event-driven, multicentre study comparing the efficacy and safety of oral rivaroxaban with placebo for reducing the risk of death, myocardial infarction or stroke in subjects with heart failure and significant coronary artery disease following an exacerbation of heart failure: the COMMANDER HF trial. Eur J Heart Fail. 2015;17(7):735-742. doi: 10.1002/ejhf.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84(408):1065-1073. doi: 10.1080/01621459.1989.10478873 [DOI] [Google Scholar]
- 19.Al-Khadra AS, Salem DN, Rand WM, Udelson JE, Smith JJ, Konstam MA. Warfarin anticoagulation and survival: a cohort analysis from the studies of left ventricular dysfunction. J Am Coll Cardiol. 1998;31(4):749-753. doi: 10.1016/S0735-1097(98)00006-0 [DOI] [PubMed] [Google Scholar]
- 20.Cokkinos DV, Haralabopoulos GC, Kostis JB, Toutouzas PK; HELAS investigators . Efficacy of antithrombotic therapy in chronic heart failure: the HELAS study. Eur J Heart Fail. 2006;8(4):428-432. doi: 10.1016/j.ejheart.2006.02.012 [DOI] [PubMed] [Google Scholar]
- 21.Cleland JG, Findlay I, Jafri S, et al. The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure. Am Heart J. 2004;148(1):157-164. doi: 10.1016/j.ahj.2004.03.010 [DOI] [PubMed] [Google Scholar]
- 22.Massie BM, Collins JF, Ammon SE, et al. ; WATCH Trial Investigators . Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119(12):1616-1624. doi: 10.1161/CIRCULATIONAHA.108.801753 [DOI] [PubMed] [Google Scholar]
- 23.Homma S, Thompson JL, Pullicino PM, et al. ; WARCEF Investigators . Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366(20):1859-1869. doi: 10.1056/NEJMoa1202299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Treasure CB, Vita JA, Cox DA, et al. Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation. 1990;81(3):772-779. doi: 10.1161/01.CIR.81.3.772 [DOI] [PubMed] [Google Scholar]
- 25.Jafri SM, Ozawa T, Mammen E, Levine TB, Johnson C, Goldstein S. Platelet function, thrombin and fibrinolytic activity in patients with heart failure. Eur Heart J. 1993;14(2):205-212. doi: 10.1093/eurheartj/14.2.205 [DOI] [PubMed] [Google Scholar]
- 26.Lip GY, Gibbs CR. Does heart failure confer a hypercoagulable state? Virchow’s triad revisited. J Am Coll Cardiol. 1999;33(5):1424-1426. doi: 10.1016/S0735-1097(99)00033-9 [DOI] [PubMed] [Google Scholar]
- 27.Gibbs CR, Blann AD, Watson RD, Lip GY. Abnormalities of hemorheological, endothelial, and platelet function in patients with chronic heart failure in sinus rhythm: effects of angiotensin-converting enzyme inhibitor and beta-blocker therapy. Circulation. 2001;103(13):1746-1751. doi: 10.1161/01.CIR.103.13.1746 [DOI] [PubMed] [Google Scholar]
- 28.Gheorghiade M, Thyssen A, Zolynas R, et al. Pharmacokinetics and pharmacodynamics of rivaroxaban and its effect on biomarkers of hypercoagulability in patients with chronic heart failure. J Heart Lung Transplant. 2011;30(2):218-226. doi: 10.1016/j.healun.2010.08.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
Statistical analysis plan
eTable. Cause-Specific Mortality by Treatment Group-GTED (Intent-To-Treat Analysis Set)
eFigure 1. Kaplan-Meier Estimates for Myocardial Infarction
eFigure 2. Kaplan-Meier Estimates for Ischemic Stroke
eAppendix. Investigator Manual for Outcome Events RIVAROXHFA3001 The COMMANDER HF Study