Table 3.
Arene Scope of the Dearomative syn-1,4-Diaminationaa
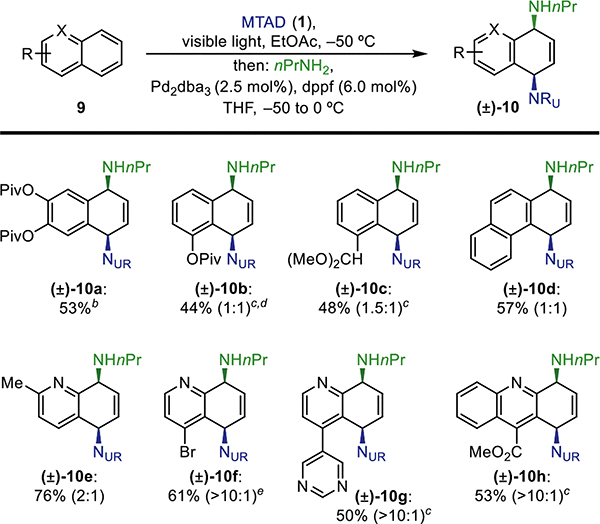 |
Standard reaction conditions: MTAD (1, 1.0 mmol, 1.0 equiv), arene (9, 2.0 mmol, 2.0 equiv), EtOAc (0.1 M), visible light, −50 °C, 12 h; then addition of nPrNH2 (2.0 mmol, 2.0 equiv) and [Pd] catalyst (5 mol %) in THF, −50 to 0 °C, 5 h. Reported yields are of isolated products, with ratios of constitutional isomers (in parentheses) determined by 1H NMR of the crude reaction mixtures.
[Pd] catalyst in THF, −20 °C, 20 h.
CH2Cl2 was used instead of EtOAc.
10 mol % of [Pd] catalyst was used.
Cycloaddition was run at 0.05 M concentration.
