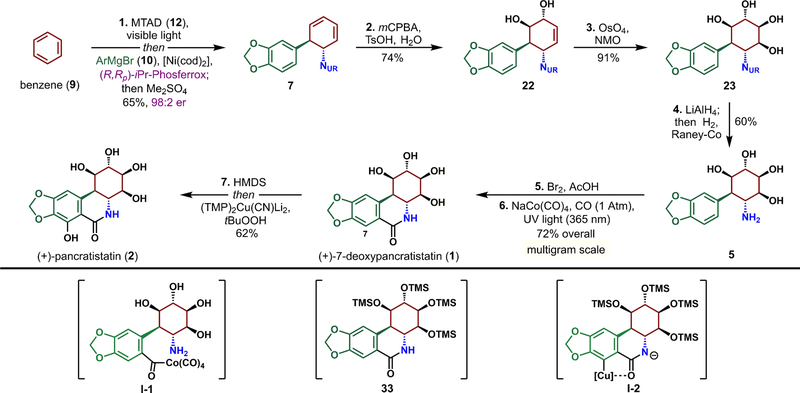
Figure 6.
Streamlined synthesis of pancratistatins 1 and 2. Reagents and conditions: 1. benzene (9), MTAD (12), CH2Cl2, visible light, −78 °C; then [Ni(cod)2] (5 mol %), (R,Rp)-iPr-Phosferrox (10 mol %), Grignard reagent 10, CH2Cl2, THF, −78 to +25 °C; then Me2SO4, K2CO3, 65% (98:2 er); 2. mCPBA, pTsOH, CH2Cl2, HFIP, H2O, 50 °C, 74%; 3. NMO, OsO4 (5 mol %), tBuOH, H2O, 25 °C, 91%; 4. LiAlH4, THF, reflux; then Rochelle salt; then Raney-Co, H2 (1 atm), 60 °C, 60%; 5. Br2, AcOH, 25 °C; 6. NaCo(CO)4 (30 mol %), nBu4NBr, CO (1 atm), NaHCO3, H2O, 1,4-dioxane, 365 nm light, 60 °C, 72% over two steps; 7. HMDS, I2 (1 mol %), MeCN, 80 °C; then solvent removal and (TMP)2Cu(CN)Li2, THF, − 78 °C → 0 °C; then tBuOOH, THF, − 78 °C; acidic workup, 62%.